Safety data, unblinded trials and COVID-19 products in breast-feeding mothers, infants and pregnant women in the United States
Updated version including VAERS data as of Sept. 15, 2023
Reminder of relevant papers:
Detection of Messenger RNA COVID-19 Vaccines in Human Breast Milk published by Hanna Nazeeh et al. in JAMA in 2022
Biodistribution of mRNA COVID-19 vaccines in human breast milk published by Hanna Nazeeh et al. in eBiomedicine (The Lancet) in 2023
There’s probably a reason why the original exclusion criteria list for the COVID-19 injectable product Phase III trials excluded breast feeding mothers and pregnant women. There’s also probably a reason we don’t want pregnant women to drink and smoke.
Exclusion Criteria: Women who are pregnant or breastfeeding.1
There is something I would like to bring attention to (and focus on) in this article: we must not lose sight of the fact that the original trials for the COVID-19 products were NOT properly executed and that all subsequent ‘trials’ are being piggy-backed off of the decisions made based on the ‘data’ from these original trials. EUA and eventual full FDA approval for these COVID-19 injectable products were granted under what most people would consider coercive and/or incentivized circumstances using absolutely anemic data.
Even in light of Brook Jackson’s testimony, since these trials had no control group due to the fact that the participants were unblinded and injected in the Moderna (by the way, when you click on the Moderna website link - ‘the page is not found’), Pfizer and Aztrazeneca trials, the data thereafter is meaningless and causation cannot be ascribed if AEs present. This is the whole point of doing a randomized controlled trial. If the participants are unblinded2 and injected during the trial, then the trial is ‘safe and effectively’ over. (Did you like that?) This is what happened: we have pathetic short term and NO long term safety data in the context of the COVID-19 products and thus no basis for declarations of these products being safe - even in the short term.
The study P203, um, as I mentioned - because of the availability of an alternate COVID-19 vaccine, uh, after a certain period of time - after basically end of May - they, we have lost the placebo group, so it’s, we cannot really say anything about, uh, the duration of vaccine efficacy - after that there is no more efficacy data, basically, after that time point. Rachel Zhang, MD, Team Leader, Clinical Review Staff, FDA
She’s right. But she didn’t address the issue of the lack of safety data. As I have said many times, if the product has not been proven to be safe, then who cares if it works.
There were some sensible people raising concerns when this ‘ethical problem’ arose, however. The sarcastic ethical problem of whether or not to inject the poor placebo participants.
Trial participants shouldn't be moved to the head of the line, said Franklin Miller, a professor of medical ethics at Weill Cornell Medical College in New York.
"That's really going to damage your trials," Caplan said, because it won't be possible to compare the two groups over a longer time period.
"The bedrock principle in human research is that there must be voluntary and informed consent," Mildred Solomon, a faculty member of the Center for Bioethics at Harvard Medical School and president of the Hastings Center, a nonpartisan bioethics think tank, said.3
The rest of this article will focus more on VAERS data pertaining to infants and mothers who were exposed to the COVID-19 injectable products. You can see my previous reports on VAERS reports for babies 0-2 here, maternal exposure to during breastfeeding reports to VAERS here, reproductive issues using Pfizer data and VAERS here, pushing COVID-19 injections on pregnant women here and here, an Epoch Times article on ‘the menstrual thing’ here, putting these products on the childhood vaccination schedule here, an OB/GYNs data here, and targeting OB/GYNs to get these things into pregnant women here and here.
The reason I started this article this way is because as time goes on, and these products are successively approved with increasingly anemic data (some involving only mouse experiments), the original exclusion criteria list is effectively being rapidly impinged upon - aka: becoming an inclusion criteria list. Meaning, young children - including infants - pregnant women, obese people, people with autoimmune conditions, etc. are not only being allowed to be injected with these products, but encouraged to do so.
Please refer to my previous article pertaining to infants and mothers exposed to COVID-19 injectable products here for the Abstract, Background and Methods.
Results as of September 15, 2023
Table 1 shows some of the MedDRA-coded adverse event (AE) types for Groups 1 (infants 0-4), 2 (pregnant women), and 3 (breast feeding women) and the total number of individuals who reported AEs (N).4 The number of types of AEs as sets of symptoms (eg: “Diarrhoea”, “Incorrect dose administered”, “Malaise”, “Productive cough”, “Rhinorrhoea”) are 1,345, 4,354 and 1,088 for groups 1-3, respectively. It is clear that the number of side effects is not low per group. Table 1 shows a sample of standalone MedDRA-coded AEs per group. Of note, the first MedDRA-coded AE in Group 1 is “No adverse event”. This is significant in my opinion and I will explain why in the following section. The total number of people who reported AEs are N = 4,502, N = 6,028 and N = 1,742 for each respective group.5
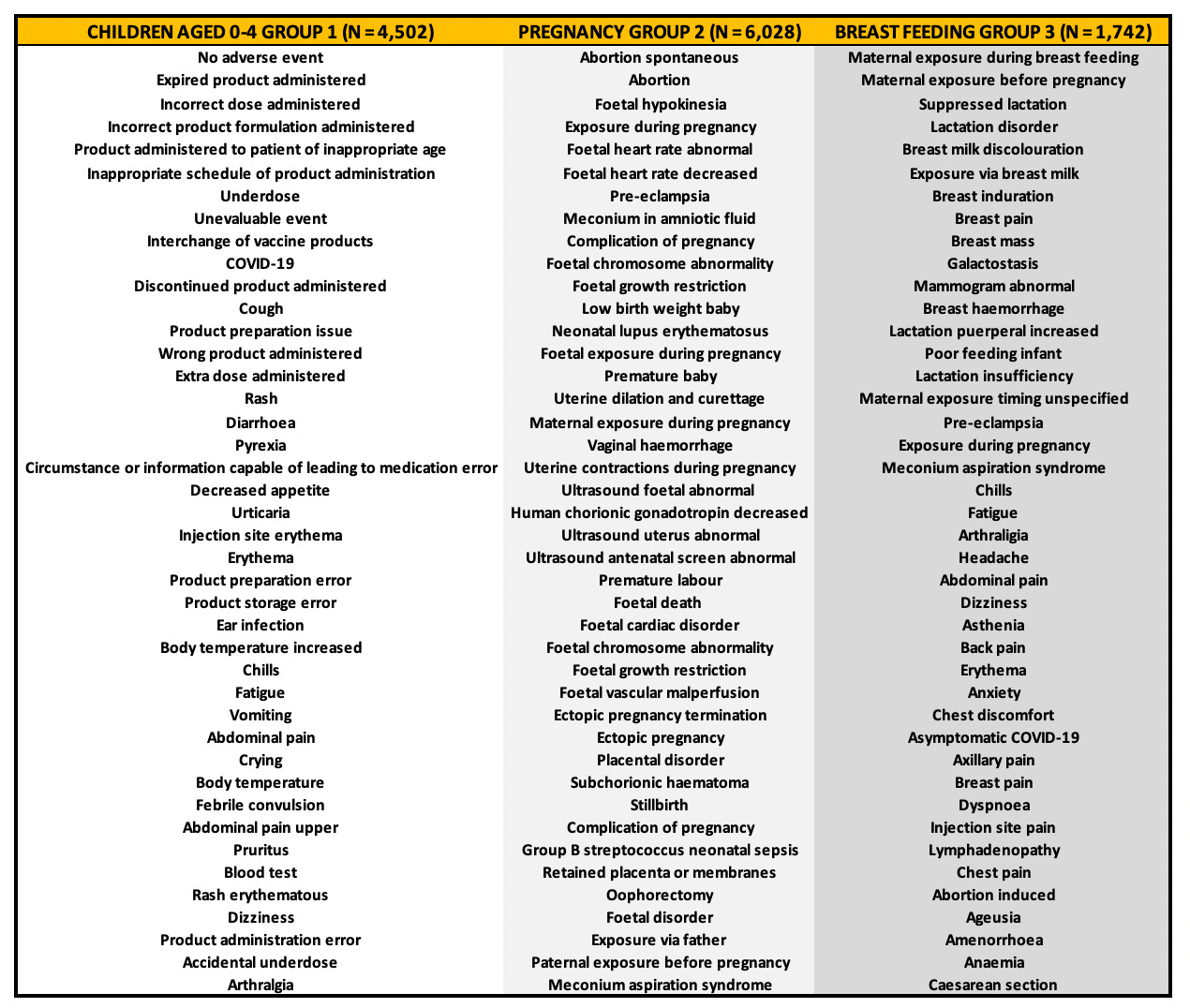
Group 1: Children aged 0-4
To date (September 15, 2023), there are 4,502 AE reports in the U.S. VAERS database for infants between 0 and 4 years of age. As mentioned, there are a total of 1,345 types of AEs as sets of symptoms for Group 1. Standalone MedDRA coded AEs range from “Incorrect product formulation administered” to “Death”, but the highest reported AE for infants was “No adverse event” (559) followed very closely by “Expired product administered” (521).
The fact that the highest reported ‘AE’ is not an actual clinical symptom but a report of non-reaction is problematic. If a VAERS report gets filed successfully and a permanent VAERS ID assigned, then that report effectively remains unchanged - even in the event of an attempt for a subsequent filing for an update. It is oftentimes the case that the primary filing requires updating, especially in the case where an individual dies, and therefore, if the VAERS report for an infant includes '“No adverse event” and that infant subsequently dies, it will more than likely not be reflected in VAERS, and certainly not in the context of the same VAERS ID.
The problem I see here is that filing a report of “No adverse event” - a non clinical outcome - precludes the reporting of an eventual AE that describes a clinical outcome and perhaps a serious one.
68% of the reports were filed immediately on site (day 0).
Please refer to this article here for statistics on infants having been injected with the COVID-19 products prior to the approval of the products. Not surprisingly, “Incorrect dose administered” and “Product administered to patient of inappropriate age” were two of the most prevalently used MedDRA codes.
The next top 8 reported AEs for infants include “Incorrect dose administered” (428), “Incorrect product formulation administered” (401), “Product administered to patient of inappropriate age” (214), “Inappropriate schedule of product administration” (203), “Underdose (176)”, “Unevaluable event (131)”, “Interchange of vaccine products” (100) and “COVID-19” (88). Notice that all but one of these MedDRA coded AEs involves a mistake made by the administrator with no indication of clinical outcome. It has been reported in the symptom text that the administrator would often forget to dilute the vial contents prior to injection of infants, effectively double dosing them. We do not know the effects of doing this.
“Vial of Covid 19 6m-4yr vaccine was not diluted with sodium chloride and all 10 doses were given to the patient in one injection.”
This was done by a pharmacist. The child apparently also had a symptomatic case of foot and mouth disease.
The table below demonstrates the differences in products based on cap color (big thanks to Maria Gutschi) and although Pfizer was asked to create color variation in caps so as not to confuse administrators, they kindly refused. Making the mistake of not diluting products and/or picking up the wrong product when you’re heading toward the thigh of a newborn however, has nothing to do with cap color. It is pure negligence, in my opinion, if such dire mistakes are made. And judging on what I have learned in the past 3 years, these mistakes happen a lot.
The split between infant sexes is 48.4% female, 49.6% male (2% unknown). Figure 1 shows the distribution of AEs by age and dose (left) and by age and sex (right). It appears at least from reports of AEs that the number of doses per age is decreasing. Now this could represent a decrease in AE occurrence or a decrease in receptivity to shooting up infants based on injury occurrences from previous doses. Which do you think is more likely?
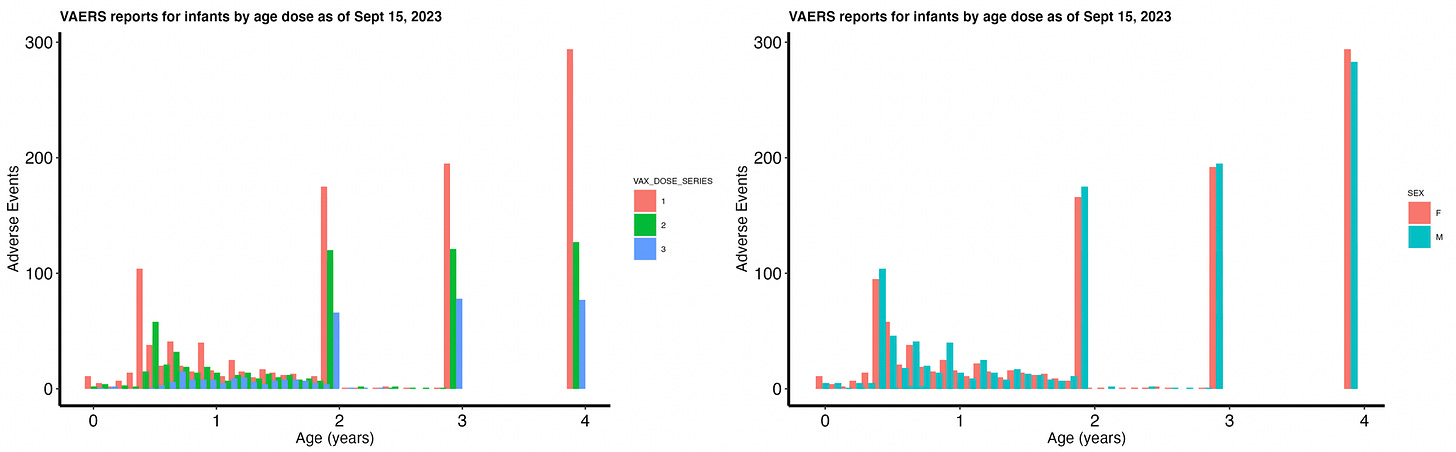
There are many reports made for infants less than 1 year old. It is preposterous to me that so many infants have been injected with these products considering that the trials upon which ‘safety’ of these products was assessed involved 8 mice.67
FDA Commissioner Dr. Robert Califf explained why the agency is moving forward without the complete human trial data.
"In the midst of a pandemic, if you wait for all that data to come in, you've missed the boat. And so you have to be preemptive," he said.8
Yes. I guess a lot of people did miss the boat. The slide below was one of two at the end of a presentation given by a Pfizer representative Kena A. Swanson, Ph.D. Vice President, Viral Vaccines Vaccine Research and Development, Pfizer Inc., to show evidence of “Immunogenicity and safety of Omicron variant-modified vaccines as a booster or primary series to support variant modified EUA”.9
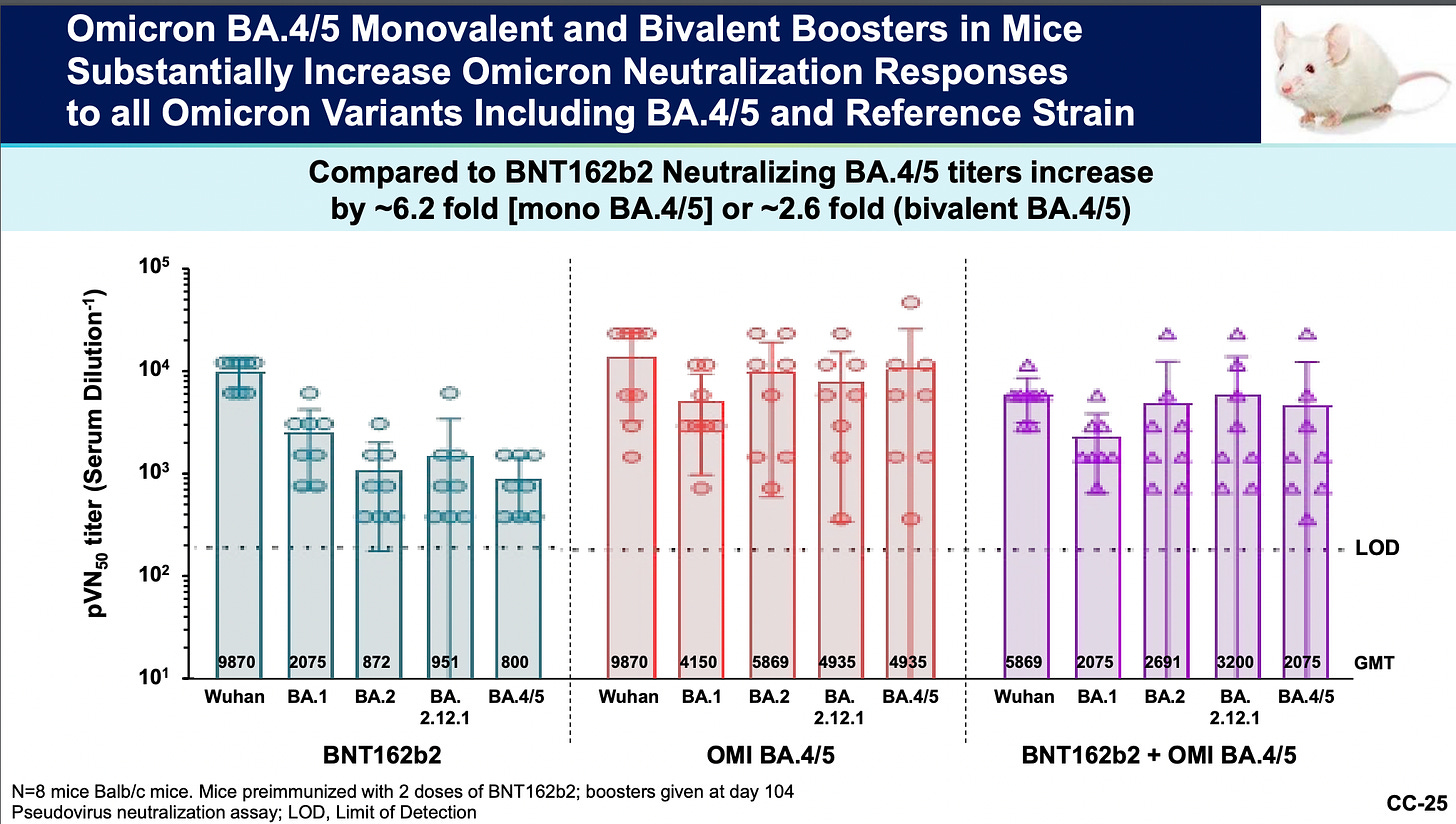
I cannot stress this point enough: all of these mediocre trials are subsequent to equally inadequate trials that were not randomized and controlled trials (RCTs). The placebo groups were unblinded and injected with product effectively intentionally losing the control groups in the original clinical trials.101112
We argue that, once proven efficacious, vaccine makers and researchers have an ethical obligation to unblind the placebo groups of COVID-19 vaccine trials and offer them vaccine, based on the four principles of medical ethics. (Ref #11)
An ethical obligation? You cannot be serious. These people don’t know what an RCT is. And if you don’t want to take my word for it. Take theirs! Start at timestamp 1:20 to hear phone call and Joseph Fraiman’s information.
We do RCTs because it’s the only decent way to find out if a drug is CAUSING adverse events observed.
Of the infants ages 0-4 with VAERS reports, 7 (0.16%) died, 67 (1.5%) were hospitalized and 226 (5%) were taken an emergency room doctor for care. 6% of the AEs are SAEs. However, of the AEs, there were 111 (2.5%) classified as cardiovascular and 7 (0.2%) as neurological which includes reports of “Basal ganglia infarction” and “Brain oedema” (swelling). Currently, there are 117 (1.9%) breakthrough COVID-19 cases.
Group 2: Pregnancy
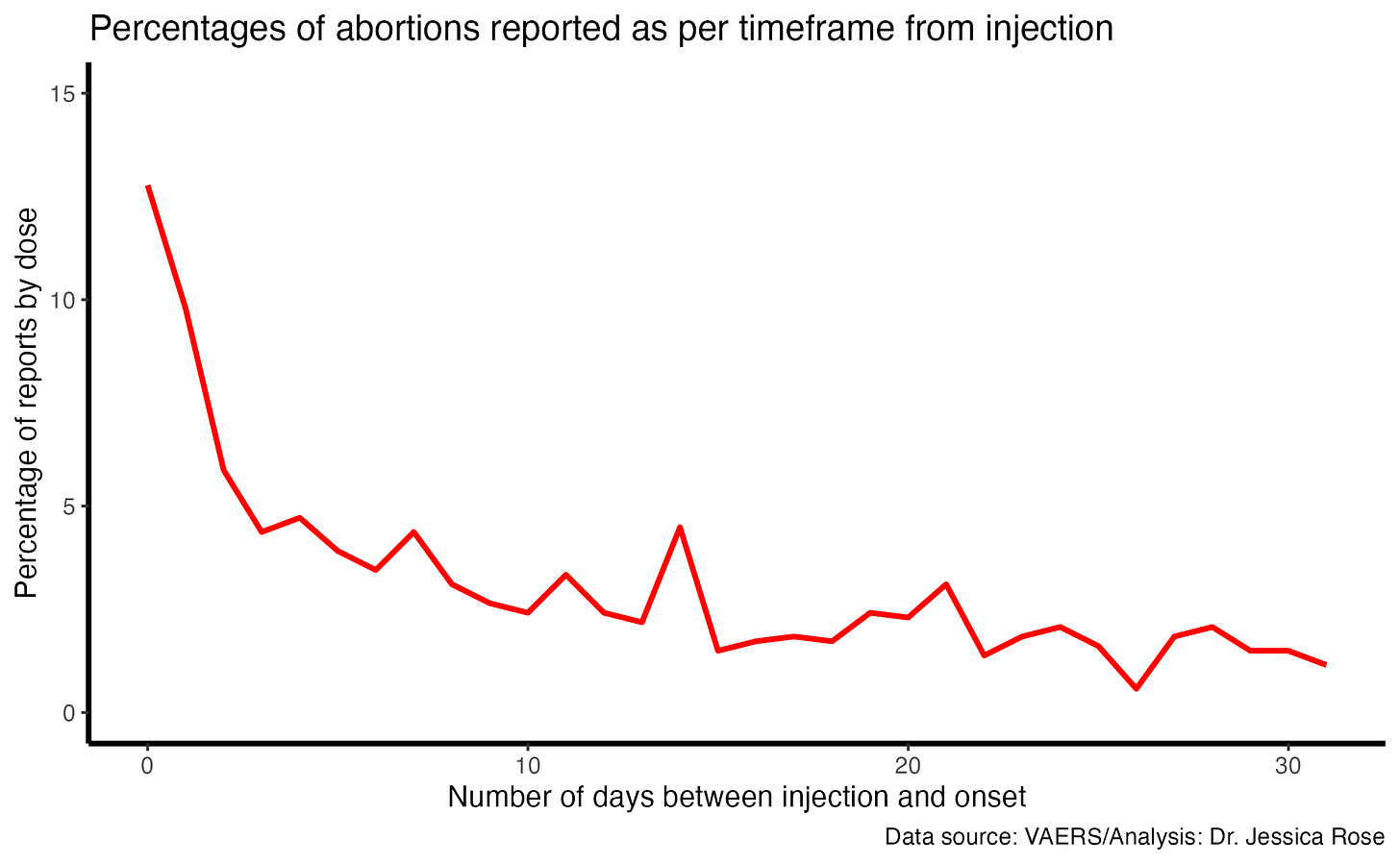
To date (September 15, 2023), there are 6,028 AE reports in the VAERS database for pregnant women. Alarmingly, 32% of the AEs are considered SAEs according to the VAERS classification system of what qualifies as an SAE. This is twice the standard according to the VAERS handbook. 22.6% of all the women who reported being pregnant and exposed to the COVID-19 products experienced and reported an abortion and 1% reported still births. The number of days following injection that the women exposed reported their pregnancies is shown below. 12.8% of women reported experiencing an abortion on the same day as their COVID-19 injection.
As mentioned, there are a total of 4,354 types of AEs as sets of symptoms for Group 2. Standalone MedDRA coded AEs range from “Chills” to “Death”, but the highest reported AE for pregnant women was “Exposure during pregnancy” (1,205) followed very closely by “Abortion spontaneous” (1129). The next top 8 reported AEs for pregnant women include “Maternal exposure during pregnancy” (560), “COVID-19” (241), “Chills” (221), “Abdominal pain” (122), “Dizziness” (100), “Blood test” (94), “Arthralgia” (89) and “Delivery” (88).
Of these women, 21 (0.3%) died, 915 (15.2%) were hospitalized and 1,119 (18.6%) were taken an emergency room doctor for care. Of the AEs, there are 1,215 (20.1%) classified as cardiovascular and 38 (0.6%) as neurological. There are currently 418 (6.9%) breakthrough COVID-19 cases.
Group 3: Breast-feeding women
To date (September 15, 2023), there are 1,742 AE reports in the VAERS database for breast-feeding women. 13 of these reports were filed for infants less than 1 year of age and of these, 12 were exposed via breast milk. The one that got injected as an infant likely also had a fully-injected mother, so this case was interesting to me. Below is his symptom text in the report. VAERS ID: 2401642
Sounds like a smart baby. Follow-up of these infants is not reported to VAERS and outcomes are generally not known.
The following is the symptom text reported for an infant who was exposed via breast milk at the age of 1. Pardon the ‘yelling’ uppercase text: it’s verbatim. VAERS ID: 1124474
MOTHER OF 12 MONTH OLD BOY RECEIVED FIRST DOSE OF COVID 19 VACCINE AT 9:15 AM SHE BREASTFED HER 12 MONTH OLD SON 3 HOURS LATER AND WHILE BREASTFEEDING THE CHILD DEVELOPED ACUTE ANAPHYLAXIS. TO BE CLEAR: MOTHER HAD THE VACCINE AND THE CHILD HAD THE REACTION.
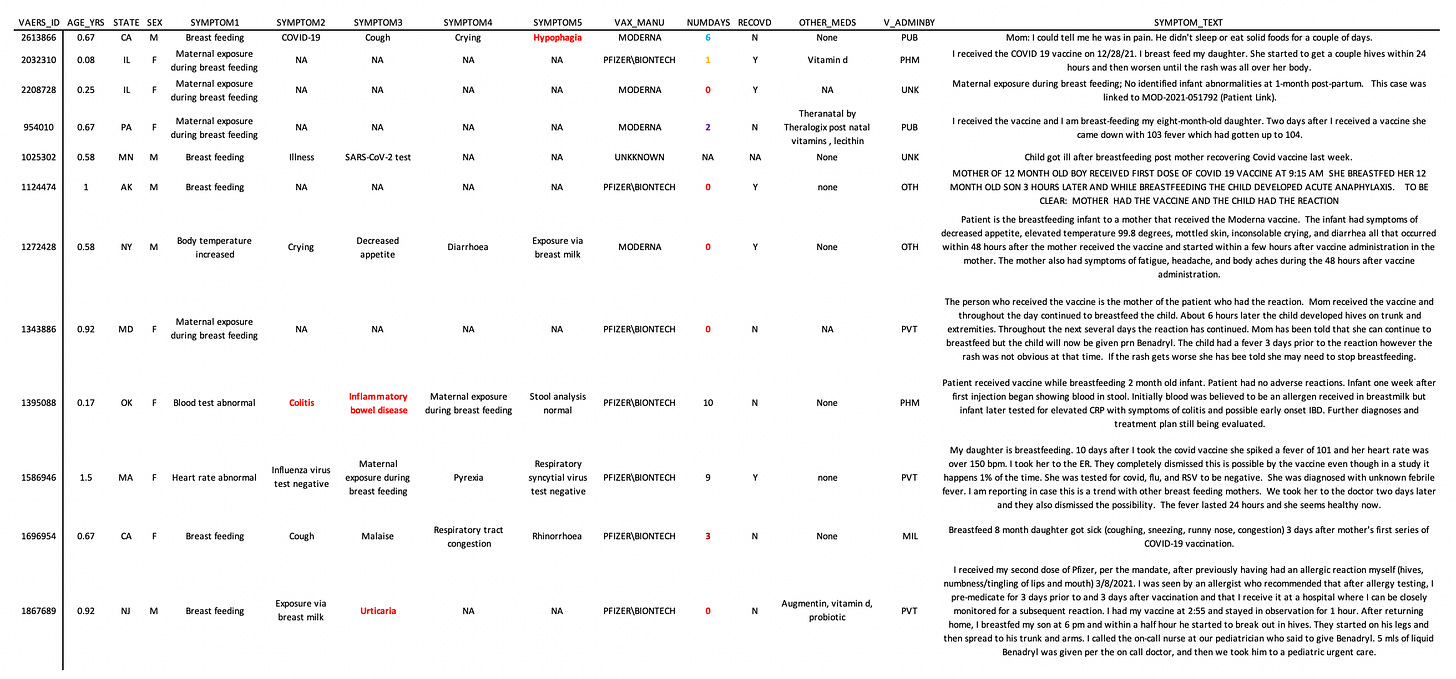
The following is a table listing some of the variables for the 12 babies exposed via breast milk. I apologize again for the small text but it was important to get as much data shown as possible. I concatenated some of the text from the symptom text variable as well.
I received the vaccine and I am breast-feeding my eight-month-old daughter. Two days after I received a vaccine she came down with 103 fever which had gotten up to 104, she has had a fever for four days (Interjection: Is there a concern of brain damage here?) now but it is down to 99, we did not have to take her to the doctor but we did call in and were told to monitor her and give her Tylenol and Motrin alternating.
As mentioned, there are a total of 1,088 types of AEs as sets of symptoms for Group 3. Standalone MedDRA coded AEs range from “Foetal growth abnormality” to “Death”, but the highest reported AE for pregnant women was “Maternal exposure during pregnancy” (560) followed very closely by “Abortion spontaneous” (115). The next top 8 reported AEs for pregnant women include “Maternal exposure during breast feeding” (101), “COVID-19” (94), “Breast feeding” (89), “Chills” (44), “Glucose tolerance test” (41), “Fatigue” (39), “Maternal exposure before pregnancy” (31), “Arthralgia” (28).
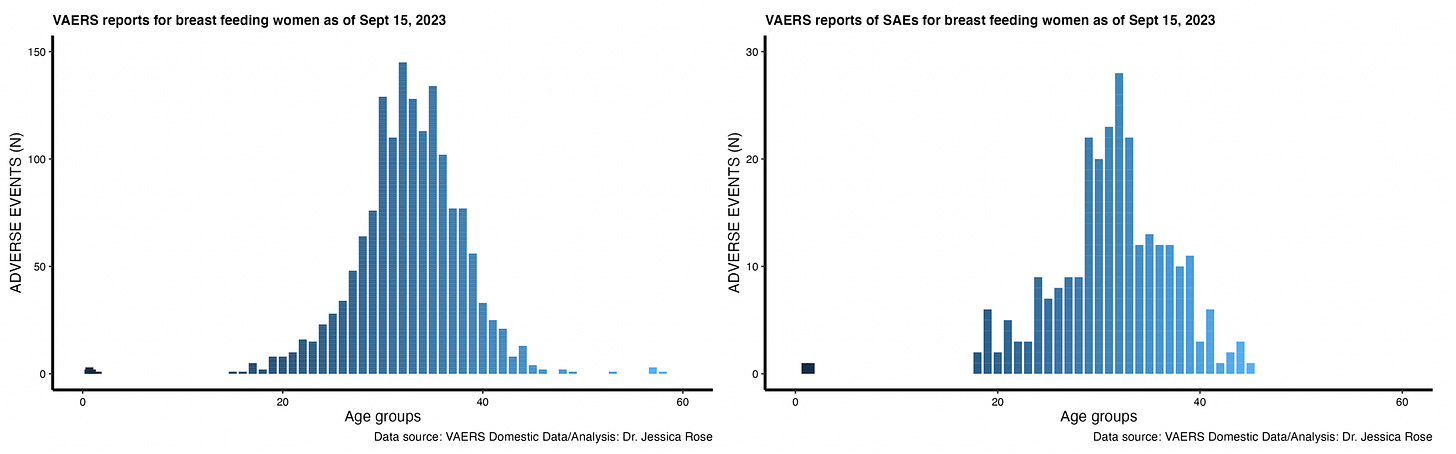
17% of the AEs reported by breast feeding mothers were considered SAEs. This is, again, higher than the standard according to the VAERS handbook. Figure 3 shows the distribution by age of AEs reported for breast feeding women with the total number of AEs on the left and the SAEs on the right. The bars in the infant range are the reports made for the infants.
Of these women, 10 (0.6%) died, 154 (8.8%) were hospitalized and 144 (8.3%) were taken an emergency room doctor for care. Of the AEs, there are 171 (9.8) classified as cardiovascular and 6 (0.3%) as neurological. There are currently 161 (9.2%) breakthrough COVID-19 cases.
Discussion Points
The total number of dead is 38 in these 3 groups of mothers and babies. This represents a small percentage of the total number of deaths in VAERS (0.3%). Although these percentages are low, it is important to note that the Group 1 demographic has more recently been added to the COVID-19 product roll-out so this data is still very early and likely to grow. Also, do not forget, that it is likely that follow-up reports will not be filed. It is also vital to recall that these data represent only a fraction of the actual AE occurrences due to known under-reporting and under-recording issues in VAERS.
Of the infants, many under the age of 18 months. 76.3% of the infants are under 3 years old. This is interesting in the context of other childhood vaccines since the schedule includes many injections at age 3 years. Investigation into co-injecting and follow-up studies are necessary.
It is disturbing that children in infancy are suffering cardiovascular and neurological AEs in temporal proximity to being exposed to these products.
It is also disturbing that 22.6% of all mothers exposed to these COVID-19 products reported having a spontaneous abortion (miscarriage).
Conclusions
Breast-feeding mothers, infants and pregnant women were in the exclusion criteria list for the phase III clinical trials of both Pfizer and Moderna. Therefore, there is no way to predict the effects on individuals in these contexts. Safety cannot be claimed for this reason and also based on the fact that the SAE reports in the VAERS database are atypically high for the groups examined.
The modified mRNA products ARE experimental: mRNA platforms are new in medical microbiology and have never before been implemented for use in human subjects on a global scale. The lipid nanoparticle (LNP) technology is also experimental. Please watch my presentation here for information pertaining to characterization of the LNPs. The fact that both the modified mRNA and the LNPs are both novel and experimental is not refutable.
Since it was not possible to perform a study on the effects of a full-term pregnancy in the timeframe lapsed for the clinical trials to have been deemed respectable and to subsequently issue EUAs for these products, it is not possible now to draw conclusions as to the effects on pregnant women or their infants who are breast-feeding, in my opinion. I don’t care how many mice smiled during antibody titer studies.
The speed-of-science way to answer questions pertaining to safety and efficacy in pregnant women and infants is to inject them all and collect the data. Seems like that’s what is being done. Also sounds like a crime against humanity. Doesn’t it?
https://clinicaltrials.gov/study/NCT04368728?a=18
Wan M, Orlu-Gul M, Legay H, Tuleu C. Blinding in pharmacological trials: the devil is in the details. Arch Dis Child. 2013 Sep;98(9):656-9. doi: 10.1136/archdischild-2013-304037. Epub 2013 Jul 29. PMID: 23898156; PMCID: PMC3833301.
https://www.usatoday.com/story/news/health/2020/12/04/vaccine-ethics-does-continuing-covid-19-trials-put-volunteers-risk/6473436002/
Group 1 includes children aged 0-4. Group 2 includes women who were pregnant, got pregnant shortly after injection with a COVID-19 product or had a complication associated with their pregnancy following injection with a COVID-19 product. Group 3 includes women who were breast-feeding following injection with a COVID-19 product and provided maternal exposure. Groups 2 and 3 were created by selection of keywords that comprise the Medical Dictionary for Regulatory Activities (MedDRA) classification name given in the VAERS database.
The keywords used for Groups 2 and 3 include ‘Abortion’, ‘Pregnancy’ and ‘Foetal’ for the former and ‘Maternal exposure’, ‘Breast feeding’, ‘Breast milk’ and ‘Lactation’ for the latter.
https://www.raps.org/news-and-articles/news-articles/2022/6/vrbpac-recommends-pfizer-moderna-covid-vaccines-fo
https://www.youtube.com/watch?v=Ixm4UmldTGQ
https://www.wfmynews2.com/article/news/verify/omicron-covid-booster-approved-from-mice-not-human-fda-cdc/83-073aa85f-19b1-4adf-8e7c-a01683ca2564
https://www.fda.gov/media/159496/download
https://www.statesman.com/story/news/healthcare/2021/01/20/austin-covid-19-vaccine-trial-participants-learn-what-they-received/4179372001/
Stoehr JR, Hamidian Jahromi A, Thomason C. Ethical Considerations for Unblinding and Vaccinating COVID-19 Vaccine Trial Placebo Group Participants. Front Public Health. 2021 Jun 24;9:702960. doi: 10.3389/fpubh.2021.702960. PMID: 34249853; PMCID: PMC8264198.
https://medicalxpress.com/news/2021-01-vaccine-trial-placebo-line-real.html




One thing bothers me, and always has - with this and all other disputed items.
If the so-called vaccine deniers who have done careful analyses of available data, like this, are wrong, why are these analyses not properly and scientifically debunked? All we get is bluster, very occasionally quoting improperly conducted trials.
There is of course a good reason they are not debunked, and that is because they are correct.
Am I wrong?
So glad to see you are emphasizing the exclusion criteria and early unblinding of the oringinal phase III press releases. For Pfizer and Moderna to call them "trials" was a mockery of us all. These simple red flags should have alerted any semi-conscious physician to the shameless fraud being perpetrated in 2020. Thank you for your work.