The spike protein, ACE-2, cysteine content and redox shifts - Part I
The power of antioxidants and what they actually do in the context of COVID-19
Prologue
This article took me a long time to write because I needed to learn, and relearn, a lot of chemistry. I am a biologist, not a chemist by ‘trade’ (I felt a little out of my ‘element’ here - hardy har har) but when it comes to living systems, so many of our fields and subject matters are integral parts of each other. It also took me extra time because my original intention - to simply review a specific journal article - got somewhat railroaded, primarily because I don’t think the article is readily ‘consumable’ by the average reader even if that reader does have a science background. I realized along the way that it wasn’t going to be as easy as summarizing points raised by the authors, and that I would have to go quite deep into the fundamentals of chemistry in order to have any chance of conveying a meaningful biological message.
For me, it’s not enough to just say ‘take antioxidants’. I want my readers to understand why they should take antioxidants, if it is suggested. I want my readers to understand what an antioxidant actually is and does. I want to understand what an antioxidant actually is and does!
My hope for this article is to pick out (and apart) the most important points made in this and other related articles, and to very clearly define why certain people fair better than others with regard to COVID-19 pathology severity (and pretty much all ‘diseases’ and disorders, including cancer) in the context of redox reactions and the environment that pre-determines such things as binding events, in the first place. I have also decided to make it a multi-part series. The amount of important information to convey is too large, and too important, for a single article.
I would also like to reiterate the importance of what Bret Weinstein and Heather Heying are conveying about the implicit danger of introducing any foreign protein via the LNP/mRNA platform due to persistent and often times severe immune reactions. However, for the purposes of this article, it is the spike protein and its ability to bind the ACE-2 receptor in humans that is the focus. For all intents and purposes, the spike protein was absolutely the worst protein that could have been selected for use in the COVID-19 injections because of what it binds. And yes, ‘they’ were well aware of this bindability and the ubiquitousness of the expression of ACE-2 in a wide variety of cell types and tissues including the testes, the heart and adipose tissue.1
Outline of primary points of Part I
Antioxidants, such as thioredoxin, downgrade COVID-19 severity by interfering with SARS-CoV-2:ACE-2 binding to prevent infection of cells
Both the SARS-CoV-2 RBD and ACE-2 proteins have redox active disulfides acted upon by antioxidants
The redox status (the balance of antioxidants and reactive species) that regulates the role of cysteine residues of the cellular environment is vital and a shift in redox status pre-determines ‘disease’ states
Background on journal article
A paper soon to be printed in September 2023 (spooky that we can read it now) entitled: “Toxicity of the spike protein of COVID-19 is a redox shift phenomenon: A novel therapeutic approach2”, was published online in Free Radical Biology and Medicine. The authors suggest that amelioration of COVID-19 effects can be achieved using specific substances such as alpha-lipoic acid, methylene blue or chlorine dioxide for their restorative effects on the Krebs cycle, as antivirals and relieving the Warburg effect.
Point 1
Antioxidants, such as thioredoxin, downgrade COVID-19 severity by interfering with SARS-CoV-2:ACE-2 binding to prevent infection of cells
Before understanding the power behind this phrase, we need to understand the words that make it up. Now I should probably start with a review of cellular respiration, aerobic respiration, the Krebs cycle, the electron transport chain and ATP production, but I have saved that detail for Part II.3 Part I will be more geared toward understanding the role of endogenous and exogenous antioxidants in maintaining redox homeostasis to prevent oxidative stress - the mothership of ‘disease’ and potentially the reason for severe spike-related pathology.
Oxidant
Oxidants are electron acceptors. The chemical element oxygen, hydrogen peroxide (H2O2) and halogens are well established oxidants. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are highly reactive chemicals formed from diatomic oxygen (O2). ROS and RNS are by-products of the normal mitochondrial metabolism of oxygen (as part of ATP production → as part of the electron transport chain → as part of the last component of aerobic respiration). Superoxide radicals (O2•-) can form when a run-off electron finds a spare oxygen during the electron transport process, and these types of ROS are convertible to other reactive species once exported from the mitochondria to damage DNA, lipids and proteins and to potentially alter cellular function.4
As with all things in nature, balance is key for proper maintenance of any system, and antioxidants play big roles in counter-balancing ROS and RNS as part of normal functioning systems.

Figure I looks terrible but it’s not so bad and the main point I want to get across by introducing it is to demonstrate the ever-present balancing act in our cells with regard to the roles of oxidants and antioxidants.
Antioxidant
An antioxidant is a compound (something made up of a bunch of identical molecules) that inhibits oxidation, ie: they prevent other things from being oxidized. Oxidation is a very obnoxious word to me. I always think it must have something to do with oxygen - and it does - but not in a complete way. Originally, oxidation implied a reaction with oxygen to form an oxide. But this is incomplete because oxidation reactions, do not always involve oxygen. A more complete definition is the following:
All processes involving the loss of electrons → the increase in the oxidation state of a chemical species.5
So oxidation is about losing electrons. And thus, antioxidation, is about inhibiting the loss of electrons. Electrons are subatomic particles with negative 1 electric charge. Whenever you hear about charges, think about balance. Negative and positive. Give and take. This is important to conceptualize at this point because of something called a redox reaction. Redox is a word that is the composite (or portmanteau - ooh la la) of the words reduction and oxidation. You can probably guess that reduction might be somewhat the opposite of oxidation - and you would be right. Reduction is about gaining electrons.
Redox is one of these amazing things found in the inherent workings of biology and chemistry that involves concepts like sharing, bonding and pairing. See? We’re not so different from ol’ Mr. redox. Or should I say, Mr. and Mrs. Redox.
Antioxidants are different from reducing agents in that the latter reduce other substances while the former prevents other substances from being reduced.
Reduction and oxidation are inextricably-linked processes that involve the donation of an electron at atomic sites from one molecule to another. The electron donor is said to be oxidized, and the electron acceptor is said to be reduced. Figure 2 shows the redox reaction between Sodium (Na) and Fluorine (F) atoms, whereby the Na atom donates an electron (has been oxidized) and the F atom accepts the electron (had been reduced).

Redox reactions are ubiquitous in nature. Examples of redox reactions are photosynthesis, fire, batteries and energy synthesis: the production of ATP via the electron transfer chain in humans, for example. They are constant and dynamic processes essential to life. One of the most important redox reactions in biology is cellular respiration whereby glucose (sugar) is oxidized to CO2, and oxygen is reduced to water. Damn, it’s so beautiful, isn’t it? I will cover this in more detail in Part II of this series.
Effectively, a redox reaction, is electron swapping for the ‘purpose’ of energy exchange whereby antioxidants balance the by-products (like ROS) of redox reactions within a cell.
Thioredoxin
Thioredoxin (Trx) is an antioxidant protein as part of a central antioxidant system in mammalian cells that involves a disulfide reductase system that regulates the dithiol/disulfide balance.6 Say that five times fast. The Trx system is essential for maintenance of a reduced environment inside cells. Cells have many sub-cellular compartments and within these sub-cellular compartments, like the endoplasmic reticulum and nucleus for example, there are different distributions of reductants and oxidants. This is to maintain respective oxidizing and reducing environments necessary for the specific functions of each compartment.7
So how does this relate to prevention of infection of cells by SARS-CoV-2? Well, it has something to do with the fact that the primary function of Trx is, in fact, the reduction of oxidized cysteine residues and the cleavage of disulfide bonds.8 To understand what this means, let’s move into disulfide bonds, thiols and the vital role of the amino acid cysteine.
Thiols and disulfide bonds/bridges
A thiol is an organosulfer compound of the form R-SH. It has sulfhydryl group (the -SH) attached to an R group. An R group in chemistry, is an abbreviation for any group in which a carbon or hydrogen atom is attached to the rest of the molecule.9 An example of a thiol is the amino acid cysteine. Another example is the wine making necessitator and antioxidant glutathione. Interestingly, one can measure the amount of cellular oxidative stress by determining the ratio of reduced to oxidized glutathione within cells.1011
A disulfide bond involves two sulfurs bound together - often called a disulfide bridge. I made a little schematic (Figure 3) to demonstrate the relationship between thiols and disulfides. There is a thick black line between these two states (reduced - left and oxidized - right) but make no mistake, these are reversible states. Disulfide bridges are covalent bonds (or cross-links) between 2 cysteines. All we need is an electron acceptor, like our buddy oxygen, for this bridge to be built.

Cysteine
The critical player in the story of this article and in life itself, is cysteine. Ah, stinky cysteine. Without you, life would be pretty boring. In fact, without cysteine, there would be no SARS-CoV-2/COVID-19 story either! Cysteine is one of two amino acids that contain stinky sulfur (the other one being methionine), but only cysteine has an ionizable thiol group. Sulfur makes cysteine both stinky and special precisely because of its amazing ability to bond with itself to form disulfide bridges between cysteines. For those of you who know a little about proteins, you’ll also remember the importance of these disulfide bridges with respect to protein folding, structure, and stability.12 This cannot be underscored enough.
Cysteines are redox sensitive due to their reductive thiol side chains.13 Cysteines are more highly reactive when their thiol side chain is in the thiolate form, i.e., deprotonated at physiological pH (S−).14 This just means the hydrogen was taken off the sulfhydryl group as part of an acid-base reaction. The thiolate group is subsequently oxidizable by ROS and RNS, leading to post-translational modifications. Basically, once the sulfurs are ‘free’, they can bind with other sulfurs to form cystine: a dimer formed when two cysteines are bound together by their sulfurs.

Cysteines can interact with ROS to produce not only disulfide bonds, but sulfinic, sulfenic and sulfonic acids, and naturally, this can disturb protein function. But, like all things in nature, they have functions as well. The ability of cysteine to adopt a variety of oxidation states makes it a key regulator of redox homeostasis and signaling.15 So, disulfide bridges are the constructive ‘endpoint’ of oxidation of cysteine, so to say, and lend to proper protein functioning. Disulfides also important in that they play roles in redox cycling and regulation of enzymes and transcription factors involved in cell signaling processes.1617
Trx reductase and its substrate Trx love reducing cysteines so you can imagine in their presence, a reducing environment would ensue → ie: not many disulfide bonds. Can you think of a sub-cellular compartment that would favor a lack of disulfide bonds? Hint: See above.
The role of antioxidants in infectivity with regard to SARS-CoV-2:ACE-2 binding
Both ACE-2 and the spike protein have a lot of cysteines. Cysteines are actually not overly abundant as amino acids in mammals, but highly conserved.1819 There are 9 cysteines in the RBD of the spike protein and 8 in the ACE-2. Each of these proteins have multiple sets of disulfide bonds to ensure their holdy-foldy-folds. The SARS-nCoV-2 receptor binding domain (RBD) shown bound to human ACE-2 in Figure 5, has 4 sets of disulfide bonds: three (C336–C361, C379–C432 and C391–C525) that stabilize the protein, and one (C480–C488) that connects the loops in the distal end of the receptor-binding motif (RBM). The RBM in the SARS-CoV-2 RBD contacts the bottom side of human ACE-2. The human ACE-2 contains a particular disulfide bond (C133-C141), on the opposite end of the RBM, as shown in Figure 4 on the right in black below.2021

Here’s a twist. Cows and pigs have a Leucine in position 133 in their ACE-2s, so there’s no disulfide bond in the ACE-2 of these animals. Instead, there’s a Leucine and a thiol group. Guess what? Cows and pigs are impervious to SARS-CoV-2. Thus, susceptibility to SARS-CoV-2 is aligned with this disulfide bond being present and in-tact, as it is in the human ACE-2.
As a point of interest, C-X-X-C motifs are important domains of metal-binding proteins and oxidoreductases.2223 ACE-2 also contains a ferredoxin (iron-sulfur protein)-like fold domain that mediates electron transfer for metabolic reactions. In fact, the active site on Trx contains a thiol in a C-X-X-C motif and this is the key to the ability of thioredoxin to reduce other proteins. Keep this in mind.
Remember what we said about antioxidants? Let’s merge into point 2.
Both the SARS-CoV-2 RBD and ACE-2 proteins have redox active disulfides acted upon by antioxidants
Antioxidants balance oxidized and reduced compounds within a cell. Redox active disulfides actively undergo redox reactions to reduce to thiols and can go back to disulfides again. This is of course determined by the reducing agents present and whether or not the environment in general is reducing or oxidizing.24
Picture this. Trx can reduce the disulfide bonds of ACE-2 to yield those sulfhydryl groups that we talked about. This renders ACE-2 effectively inactive due to the fact that it cannot correctly fold without its disulfide bonds intact. Therefore, by the action of the Trx system, SARS-CoV-2 can be prevented from binding and infecting of cells. This is illustrated in Figure 6 below in a diagram extracted from reference 20 wherein the authors conclude that older individuals and/or individuals with pre-existing conditions or ‘disease states’, have increased vulnerability to SARS-CoV-2 because of augmented oxidative stress.
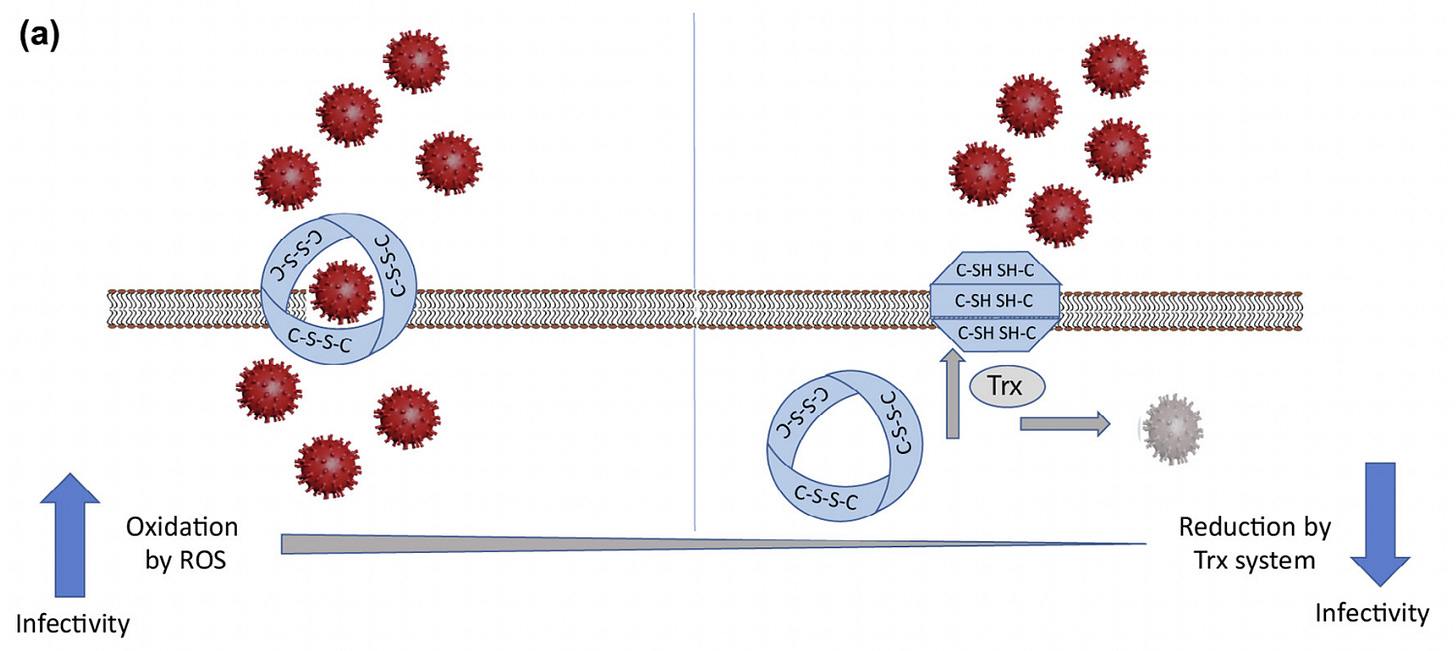
It has been shown in this particular paper (reference 20), and also is the meat of the original paper I had intended to review (reference 2), that COVID-19 pathogenesis and severity is dependent on redox since the interacting parts of the ACE-2 receptor (the ferrodoxin-like fold domain) and the spike protein, have redox-active disulfides, as mentioned. Interestingly, ferredoxin is known to pass electrons to thioredoxin which, in turn, reduces the disulfide bonds.
So this is the crux of the story. Since there is a dependency on intact disulfide bonds for proper ACE-2:spike binding, this can be exploited, so to say, to minimize binding and subsequent infection of cells. Specifically, Trx and the Trx system can act on the disulfide bonds of ACE-2 to abrogate entry of SARS-CoV-2. That is, if the reactive disulfide bonds in ACE-2 are reduced, the protein is not in the proper conformation to bind SARS-CoV-2. So, binding does not ensue and infection does not occur. Evidence in support of this lies in the fact that cows and pigs are impervious to SARS-CoV-2. In addition, the Trx system can also act directly on SARS-CoV-2 to effectively dissolve it via the same mechanism: the disulfide bonds are reduced so that the spike proteins are not in the proper conformation to bind ACE-2.
Here’s the problem, and why some people are faring very badly with SARS-CoV-2 and well, a lot of things: most people are effectively antioxidant deficient and have an excess of ROS and RNS having a party, party, party. This is precisely oxidative stress and as I will describe in Part II, oxidative stress is associated with a plethora of ‘diseases’. Again, when the balance of antioxidants and ROS or RNS is disrupted, by an excess or deficiency of either one, the human body experiences a strong redox shift, commonly referred to as none other than oxidative stress.25
And now we can slide into point 3.
The redox status (the balance of antioxidants and reactive species) that regulates the role of cysteine residues of the cellular environment is vital and a shift in redox status pre-determines ‘disease’ states
Redox shift
A redox shift is a shift in the balance between oxidized and reduced compounds within a cell (See reference 3). Under ‘normal’ conditions, a healthy cell maintains this balance via endogenous antioxidants that counteract the continuous production of ROS that is a common by-product of metabolism. Imagine that if you have a deficiency in antioxidants, you mightn’t be able to remove enough ROS and RNS and then Houston, you’ll start to have problems. What kind of problems? Well, in addition to aging (well, at least some people think of aging as a problem), disease states related to inflammation and metabolic disorders. This is because there is a decline in the thiol/disulfide equilibrium which is what we’ve been talking about this whole time. Redox imbalance can be measured by serum protein thiols and can be used to predict clinical disease.26 So if you are interested in knowing if you’re redox imbalanced, go get your serum protein thiols measured.
Perhaps the best way to demonstrate the ongoing and changing redox state with respect to aging and disease, for example, is with a picture. In the schematic below in Figure 7, on the top left, you can see the oxidized and reduced states of glutathione (as an example thiol/disulfide couple), whereby the reduced state has a thiol attached and the oxidized state involves a disulfide bridge.

In the context of the original paper, the authors describe an environment where there is a decline in the thiol/disulfide equilibrium as per aging, and that this leads to an increased susceptibility to COVID-19. In the context of the schematic above, you can imagine that someone with COVID-19 (or cancer) might have an Oxidation/Reduction potential (ORP) that is higher than someone without these conditions. So if we were to point to a spot on the red trajectory where someone with severe COVID-19 would lie in terms of OP (Oxidation Potential), it would likely be toward the top of the trajectory. What this necessarily means is that this person would likely have more sulfur molecules in the oxidized state (ie: a higher ratio of disulfides to dithiols (reduced serum protein thiols) indicating that antioxidant effects are suboptimal.
It is at this point that I leave you as I continue to work on Part II. Please, if you are a chemist, and you see that I have gotten something wrong, let me know in the comments.
The most important message I think I can convey in this Part I, is the value of antioxidants - both as endogenous and exogenous agents. We will always metabolize oxygen, and so we will always have free radicals, ROS and RNS. And perhaps as we age, the balance of the endogenous antioxidants will result in redox modulation. There’s nothing much we can do about that. But we can help ourselves stay vital and healthy (balanced) by maintaining a supply of exogenous antioxidants from food sources to counterbalance the damages induced by these normal, albeit harmful by-products. Luckily, some of the yummiest foods like blueberries and chocolate are incredibly high in antioxidants. So eat up beehatches! This is the ‘Blueberry Baby’ Summer Simpson from Nova Scotia.
Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020 Apr 28;9(1):45. doi: 10.1186/s40249-020-00662-x. PMID: 32345362; PMCID: PMC7186534.
Schwartz L, Aparicio-Alonso M, Henry M, Radman M, Attal R, Bakkar A. Toxicity of the spike protein of COVID-19 is a redox shift phenomenon: A novel therapeutic approach. Free Radic Biol Med. 2023 Jun 29;206:106-110. doi: 10.1016/j.freeradbiomed.2023.05.034. Epub ahead of print. Erratum in: Free Radic Biol Med. 2023 Jul 27;207:226. PMID: 37392949.
Fraunberger EA, Scola G, Laliberté VL, Duong A, Andreazza AC. Redox Modulations, Antioxidants, and Neuropsychiatric Disorders. Oxid Med Cell Longev. 2016;2016:4729192. doi: 10.1155/2016/4729192. Epub 2015 Nov 10. PMID: 26640614; PMCID: PMC4657108.
Matsuzaki S, Szweda PA, Szweda LI, Humphries KM. Regulated production of free radicals by the mitochondrial electron transport chain: Cardiac ischemic preconditioning. Adv Drug Deliv Rev. 2009 Nov 30;61(14):1324-31. doi: 10.1016/j.addr.2009.05.008. Epub 2009 Aug 26. PMID: 19716389; PMCID: PMC2789306.
https://en.wikipedia.org/wiki/Redox
Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014 Jan;66:75-87. doi: 10.1016/j.freeradbiomed.2013.07.036. Epub 2013 Jul 27. PMID: 23899494.
Smith KA, Waypa GB, Schumacker PT. Redox signaling during hypoxia in mammalian cells. Redox Biol. 2017 Oct;13:228-234. doi: 10.1016/j.redox.2017.05.020. Epub 2017 May 31. PMID: 28595160; PMCID: PMC5460738.
https://en.wikipedia.org/wiki/Thioredoxin
https://www.chem.ucla.edu/~harding/IGOC/R/r_group.html
Pastore A, Piemonte F, Locatelli M, Lo Russo A, Gaeta LM, Tozzi G, Federici G (August 2001). "Determination of blood total, reduced, and oxidized glutathione in pediatric subjects". Clinical Chemistry. 47 (8): 1467–1469. doi:10.1093/clinchem/47.8.1467.
Lu SC (May 2013). "Glutathione synthesis". Biochimica et Biophysica Acta (BBA) - General Subjects. 1830 (5): 3143–3153. doi:10.1016/j.bbagen.2012.09.008.
William J. Wedemeyer, Ervin Welker, Mahesh Narayan, Harold A. Scheraga. Biochemistry 2000, 39, 15, 4207–4216. Publication Date:March 21, 2000. https://doi.org/10.1021/bi992922o.
Poole LB. The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med. 2015 Mar;80:148-57. doi: 10.1016/j.freeradbiomed.2014.11.013. Epub 2014 Nov 27. PMID: 25433365; PMCID: PMC4355186.
Fra A, Yoboue ED and Sitia R (2017) Cysteines as Redox Molecular Switches and Targets of Disease. Front. Mol. Neurosci. 10:167. doi: 10.3389/fnmol.2017.00167.
Alcock LJ, Perkins MV, Chalker JM. Chemical methods for mapping cysteine oxidation. Chem Soc Rev. 2018 Jan 2;47(1):231-268. doi: 10.1039/c7cs00607a. PMID: 29242887.
Leonard SE, Carroll KS. Chemical 'omics' approaches for understanding protein cysteine oxidation in biology. Curr Opin Chem Biol. 2011 Feb;15(1):88-102. doi: 10.1016/j.cbpa.2010.11.012. Epub 2010 Dec 3. PMID: 21130680.
Maryam Ghasemitarei, Angela Privat-Maldonado, Maksudbek Yusupov, Shadi Rahnama, Annemie Bogaerts, Mohammad Reza Ejtehadi. Effect of Cysteine Oxidation in SARS-CoV-2 Receptor-Binding Domain on Its Interaction with Two Cell Receptors: Insights from Atomistic Simulations. Journal of Chemical Information and Modeling 2022, 62 (1) , 129-141.
Sun MA, Zhang Q, Wang Y, Ge W, Guo D. Prediction of redox-sensitive cysteines using sequential distance and other sequence-based features. BMC Bioinformatics. 2016 Aug 24;17(1):316. doi: 10.1186/s12859-016-1185-4. PMID: 27553667; PMCID: PMC4995733.
Attila Miseta , Peter Csutora, Relationship Between the Occurrence of Cysteine in Proteins and the Complexity of Organisms, Molecular Biology and Evolution, Volume 17, Issue 8, August 2000, Pages 1232–1239, https://doi.org/10.1093/oxfordjournals.molbev.a026406
Singh J, Dhindsa RS, Misra V, Singh B. SARS-CoV2 infectivity is potentially modulated by host redox status. Comput Struct Biotechnol J. 2020;18:3705-3711. doi: 10.1016/j.csbj.2020.11.016. Epub 2020 Nov 20. PMID: 33250972; PMCID: PMC7678423.
Lan, J., Ge, J., Yu, J. et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581, 215–220 (2020). https://doi.org/10.1038/s41586-020-2180-5.
Shuber, A. P., E. C. Orr, M. A. Recny, P. F. Schendel, H. D. May, N. L. Schauer, and J. G. Ferry. 1986. Cloning, expression, and nucleotide sequence of the formate dehydrogenase genes from methanobacterium formicicum. J. Biol. Chem.261:12942–12947.
Ammendola, S., C. A. Raia, C. Caruso, L. Camardella, S. D'Auria, M. De Rosa, and M. Rossi. 1992. Thermostable NAD(+)-dependent alcohol dehydrogenase from Sulfolobus solfataricus: gene and protein sequence determination and relationship to other alcohol dehydrogenases. Biochemistry31:12514–12523.
Hati S, Bhattacharyya S. Impact of Thiol-Disulfide Balance on the Binding of Covid-19 Spike Protein with Angiotensin-Converting Enzyme 2 Receptor. ACS Omega. 2020 Jun 23;5(26):16292-16298. doi: 10.1021/acsomega.0c02125. PMID: 32656452; PMCID: PMC7346263.
H. Sies, “Biochemistry of oxidative stress,” Angewandte Chemie International Edition in English, vol. 25, no. 12, pp. 1058–1071, 1986.
Banne, A. F., Amiri, A., & Pero, R. W. (2003). Reduced Level of Serum Thiols in Patients with a Diagnosis of Active Disease. Journal of Anti-Aging Medicine, 6(4), 327–334. doi:10.1089/109454503323028920.





Interesting piece which I must go over more slowly later today.
I always hate when they refer to supplements of natural substances as "drugs". They are not a drug IMO. Drugs for the most part interfere or block enzymatic processes, or some process. Supplements "assist" with a process. A biochemical lesion is corrected with a dose of a vitamin as an example. My lysosomal storage disorder is assisted by Arginine Supplements which are not drugs, it's an amino acid. Ok I'm off the soapbox.
The Canadian government is proposing to "regulate" natural products. Local drugstores- Shoppers, London Drugs, Pharmasave,etc.- removed NAC from their supplements a couple of years ago. It is still available at Vitamin shops. New Zealand has similar legislation. (Are we seeing a pattern? Both ruled by WEF minions.)
As NAC is a primary source of absorbable cysteine, we can connect the chemistry you have outlined with the intent to retain the spike protein pathology. Annual "boosters" are "recommended" in Canada, with B.C.'s draconian Bill 36 about to enforce injection for all licensed health care personnel.
Thank you for this review.