Please refer to my previous article entitled: “Is SARS-nCoV-2-associated systemic micro-clotting due to spike protein-induced hemolysis resulting in amyloid plaque formation?”.
Then refer to a new publication entitled: “CD147 contributes to SARS-CoV-2-induced pulmonary fibrosis” published on November 25, 2022 in Nature Signal Transduction and Targeted Therapy. This is a published article which means it’s been peer-reviewed.
In this work, the authors exemplified our dear model mouse friends again. In their model, the mice were ‘humanized’; having been granted the human versions of CD147 (hCD147), thus making them susceptible to cell infection via CD147/spike protein binding by SARS-CoV-2, complete with symptoms! Or if you prefer: “SARS-CoV-2- and its delta variant-infected humanized CD147 transgenic mice”.
Here’s their conclusion:
In conclusion, we demonstrated that CD147 contributed to SARS-CoV-2-triggered progressive pulmonary fibrosis and identified CD147 as a potential therapeutic target for treating patients with post-COVID-19 pulmonary fibrosis.
As most of you know by now, in severe cases of COVID-19, for reasons as yet to be meticulously defined, severe lung pathologies can develop including pulmonary fibrosis. Pulmonary (lung) fibrosis (development of connective tissue as a repair function due to injury - which is normal) is very serious for the same reason myocardial scarring (or any pathological tissue scarring for that matter) is very serious. Inevitably, prevention of normal functioning of the tissue/organ - due to the replacement of normal cells or tissues with scar tissue - ensues.
Imagine if you replaced the rubbery surface of a balloon with sections or grafts of inflexible cardboard and then tried to blow up the balloon. It would pop because the paper is not flexible. Connective tissue formation is absolutely normal - essential, in fact - to our normal functioning. I mean, what would we do if we didn’t have connective tissue? We would simply fall apart! Like the narrative is doing!
In all complex systems, problems arise when normal functions go straight up pathological. In the case of pulmonary fibrosis, unregulated production of connective tissue in the lungs due to extended inflammatory reactions leads to lung malfunction and death. If you want a longish lesson in connective tissue, watch the following YouTube video.
Autopsies are the best way to expose fibrotic lesions or fibrosis in any organ, and in the context of COVID-19 deaths, many other organs have been found to be fibrous-y as well.123 Too bad we are limited in our abilities to do autopsies in the context of COVID-19, eh?
Just to remind you, fibrosis is the activity of becoming fibrous and it happens due to normal functioning. Again, it is the development of connective tissue as a repair function due to injury. When it gets out of control, that’s when problems arise.
So the neat thing that the authors of this new study found, was that in addition to CD147 being a receptor for receptor-mediated infection and an inflammatory pathway promoter, it can act as a signal transducer and a fibroblast activator which is associated with pulmonary fibrosis.
Here, we identified a novel function of CD147 contributing to fibroblasts activation in COVID-19 pulmonary fibrosis besides mediating entry of virus and inducing cytokine storm.
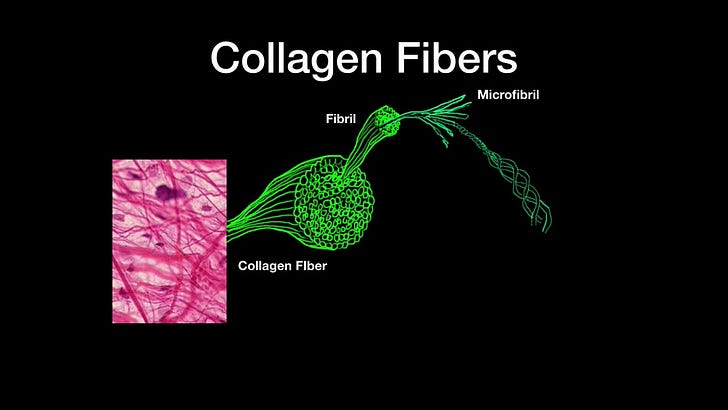
Fibroblasts are cells that define the matrix. The matrix is everywhere. It is all around us. Even now, in this very room. You can see it when you look out your window or when you turn on your television… Sorry. Glitch in the what now?
Fibroblasts synthesize extracellular matrices and collagen and are vital to wound healing. They hitchhike to wound sites to provide the material to rebuild injured sites. Collagen is the main structural protein found in these extracellular matrices. So without fibroblasts, we’d be pretty floppy beings! They are cells that provide the framework of ‘flexible solidity’, if I may, in, and throughout our bodies.
I found this video made by the John O'Neill group showing faster migration of fibroblasts to wound site during the day, as opposed to night, and it is fascinating!
Fibroblasts migrate or move due to signals from special cytokines called chemokines. Chemokines - from Ancient Greek χῠμείᾱ (khumeíā) 'alchemy', and κῑ́νησῐς (kī́nēsis) 'movement'4 - are small proteins that tell cells where to go. This is called chemotaxis. You can remember this because of the word 'taxis'. They also play vital roles in cell signaling5, infection control6 and wound healing7.8
So what’s new here is that in addition to the established functions of CD147 as a receptor for cell entry/infection and also cytokine storm mediators, they actually act as critical regulators of fibroblast activation in the SARS-CoV-2 context. The implications are quite staggering since they propose that a positive feedback loop - that has been identified in liver fibrosis and hepatocellular carcinoma - may also play a role in the context of lung fibrosis mediated by fibroblast activation.910 This positive feedback loop involves none other than TGF-β (and CD147), of course. You can read a little more about TGF-β here and here and here (if you like Wikipedia).11
During the initiation of the pulmonary fibrotic phase, epithelial cell injury and the activation of inflammation promote the production of TGF-β, which plays a critical role in the process by which fibroblasts differentiate into myofibroblasts, produce collagen, reduce lung elasticity and impair respiratory function.
The authors refer to TGF-β as the master regulator of organ fibrosis. Nice. So what this means is that there’s a system of fiber formation that once initiated, keeps itself going. Not good for lungs.
Another very important finding by these authors which validates their primary finding, is that when they 'knocked out' the CD147 receptor in the mice (this means they got rid of it using a bunch of neat lab techniques), they saw 'alleviation of accumulation of collagen fibrils' in the lungs of the mice. They also tested their findings by blocking the hCD147 receptor in the mice using a humanized anti-CD147 antibody called meplazumab.12 What they found was that the introduction of this antibody relieved "the pathological changes of pulmonary fibrosis characterized by alveolar septal thickening and pulmonary consolidation". In other words, they were able to stop SARS-CoV-2-induced pulmonary fibrosis in their mouse model using these antibodies. It is interesting that this antibody has also been found to reduce lymphopenia induced by COVID-19 as well.13
They suggest that this is a way forward for preventing extensive fibrotic damage to lung tissue in humans with COVID-19. They also checked their SARS-CoV2-pulmonary-fibrosis mouse model itself, you know, the one with the human CD147 receptor, using a genetic profiling technique called RNA-sequencing (RNA-seq) to prove that the "hCD147 mouse model with SARS-CoV-2 infection showed fibrotic transcriptional signatures similar to those associated with classical pulmonary fibrosis". So not only their finding about the therapeutic antibody was important, but the establishment of their mouse model as well.
So there you have it folks. CD147 is implicated in yet another way in the context of severe COVID-19 pathologies involving fibrosis and the excessive formation of disseminated scar tissue.
Don’t forget to remember the articles I wrote about amyloids and fibrin-related stuff.
What about in the context of the COVID-19 shots? Don’t ever forget that the binding site for CD147 sits on the end of the spike protein…
There’s no crystal structure for SARS-CoV-2 spike/CD147 so here’s a published docking simulation result with a mock-up of where CD147 would be in relation to the entire spike protein. (From reference #13)
More soon…
I just had to add here that I am working on writing up a kind of unifying theory for the amyloid idea. My feeling is that we can probably analogize here between blood clots with regard to location: stroke in the brain, pulmonary embolism in the lungs, deep vein thrombosis in the leg, etc… they are called different things but it is the same etiology: a blood clot. I propose that pulmonary fibrosis, myocarditis, and those big white proteinaceous things coming out of cadavers similarly share etiology: out of control fibrin production.141516
Wu, J., Chen, L., Qin, C. et al. CD147 contributes to SARS-CoV-2-induced pulmonary fibrosis. Sig Transduct Target Ther 7, 382 (2022). https://doi.org/10.1038/s41392-022-01230-5.
Barisione, E. et al. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 478, 471–485 (2021).
Mehta, P. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 (2020).
https://en.wikipedia.org/wiki/Chemokine
Murdoch C, Finn A (May 2000). "Chemokine receptors and their role in inflammation and infectious diseases". Blood. 95 (10): 3032–43. doi:10.1182/blood.V95.10.3032.010k17_3032_3043
Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P (December 1995). "Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells". Science. 270 (5243): 1811–1815.
Raman, Dayanidhi; Sobolik-Delmaire, Tammy; Richmond, Ann (2011-03-10). "Chemokines in health and disease". Experimental Cell Research. 317 (5): 575–589. doi:10.1016/j.yexcr.2011.01.005
Hogaboam CM, Steinhauser ML, Chensue SW, Kunkel SL. Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney Int. 1998 Dec;54(6):2152-9. doi: 10.1046/j.1523-1755.1998.00176.x. PMID: 9853282.
Li, H. Y. et al. Activation of TGF-beta1-CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis. Sci. Rep. 5, 16552 (2015).
Wu, J. et al. Regulation of a TGF-beta1-CD147 self-sustaining network in the differentiation plasticity of hepatocellular carcinoma cells. Oncogene 35, 5468–5479 (2016).
Wiseman DM, Polverini PJ, Kamp DW, Leibovich SJ. Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun. 1988 Dec 15;157(2):793-800. doi: 10.1016/s0006-291x(88)80319-x. PMID: 2462419.
https://go.drugbank.com/drugs/DB16465
Helal MA, Shouman S, Abdelwaly A, Elmehrath AO, Essawy M, Sayed SM, Saleh AH, El-Badri N. Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia. J Biomol Struct Dyn. 2022 Feb;40(3):1109-1119. doi: 10.1080/07391102.2020.1822208. Epub 2020 Sep 16. PMID: 32936048; PMCID: PMC7544927.
Imokawa S, Sato A, Hayakawa H, Kotani M, Urano T, Takada A. Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med. 1997 Aug;156(2 Pt 1):631-6. doi: 10.1164/ajrccm.156.2.9608094. PMID: 9279250.
Schnitt SJ, Stillman IE, Owings DV, Kishimoto C, Dvorak HF, Abelmann WH. Myocardial fibrin deposition in experimental viral myocarditis that progresses to dilated cardiomyopathy. Circ Res. 1993 Apr;72(4):914-20. doi: 10.1161/01.res.72.4.914. PMID: 7680288.
Kell DB, Laubscher GJ, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 2022 Feb 17;479(4):537-559. doi: 10.1042/BCJ20220016. PMID: 35195253; PMCID: PMC8883497.




Research on CD147 may be very helpful in the long run. I am a retired physician and am aware of studies showing that aspirin will slow down progression of emphysema. Perhaps the stimulation of fibroblasts is due to local micro-clotting attracting fibroblasts by way of signals from platelets aggregating at these sites.
So my first question is: Does aspirin, Plavix, heparin, or other anti-platelet/anticoagulant-class medications used in hospitalized COVID-19 patients reduce the chance of pulmonary fibrosis?
My second question is: Do the new endothelial cell protective agents such as Flavicin and Salifen have benefit in such patients?
Lastly, there is a relatively inexpensive medication/(extract) named Pynogenol used in preventing recurrent venous thrombosis in patients with lower extremity venous insufficiency and more to the point, good studies showing it is protective in diabetic retinal micro-vascular disease. If I were a patient suffering from progressive COVID-induced pulmonary fibrosis, I would certainly look into these types of options.
Stopping pulmonary fibrosis is certainly worthwhile. In hepatic fibrosis there is regeneration of tissue if the cause is removed- the structure may not be perfect, but the function is pretty good.
Reversal of pulmonary fibrosis is usually not seen; Kaplan-Meier nomograms for pulmonary function with age show a consistent decline. For instance, quitting smoking does not restore the lung function to the baseline for age, but the slope of the decline may become parallel to the "normal" for age. As in myocarditis, the damage from pulmonary fibrosis should be seen as permanent.
It would be good to put the mice through another study comparing proper early treatment to untreated Covid infection to mRNA injection.