By this time next year (at the latest), they will admit that their products were insufficiently safety tested. They will have to. It's not deniable.
Because they were insufficiently safety tested.
Please head to the TGA’s Delegate’s Overview and Request for ACV’s Advice from January 11, 2022 regarding the p-fizer/comirnaty product. It took place at The 18th ACV meeting on 15 January 2021.
The clinical data to support this provisional registration are largely from Study C4591001, an ongoing Phase 1/2/3,Page 4 of 53 randomized, observer-blind, placebo-controlled study to assess immunogenicity, efficacy and safety of BNT162b2 [mRNA] vaccine.
This study involved 16 year olds and older, 38,000 individuals with 2 months follow-up with claimed mild or moderate reactogenicity, low incidence of serious adverse events, and no clinically significant safety concerns. Remember that.
Limitations of the current data include:
1. Safety follow up is currently limited to median two months post Dose 2
2. The duration of immune response and vaccine protection is not currently known
3. Vaccine efficacy against asymptomatic infection and viral transmission are not yet known
4. The data in immunocompromised individuals are very limited
5. Lack of data in paediatric subjects, pregnant women, and lactating mothers
It is important to mention that they simply ‘proposed’ that these ‘limitations’ should be addressed.
I also find it interesting that the box in the following screenshot from page 4 is not checked. Doesn’t that mean, that there was a reason to say, at that time, that the application for Comirnaty should not be approved for provisional registration?
I would like to make one more note about the ‘data limitations’ from this ‘study’, and a note on the AEs and pregnancy information. A correlate of protection has not been established. So, you haven’t, after over a year of in vivo trials in humans, been able to establish that it provides protective immunity. SO WHY SHOULD ANYONE TAKE IT?
Pregnant women or to be expecting women should know what the updated data clearly showed and are reported here. The recommendation is that it should not be taken by pregnant women.
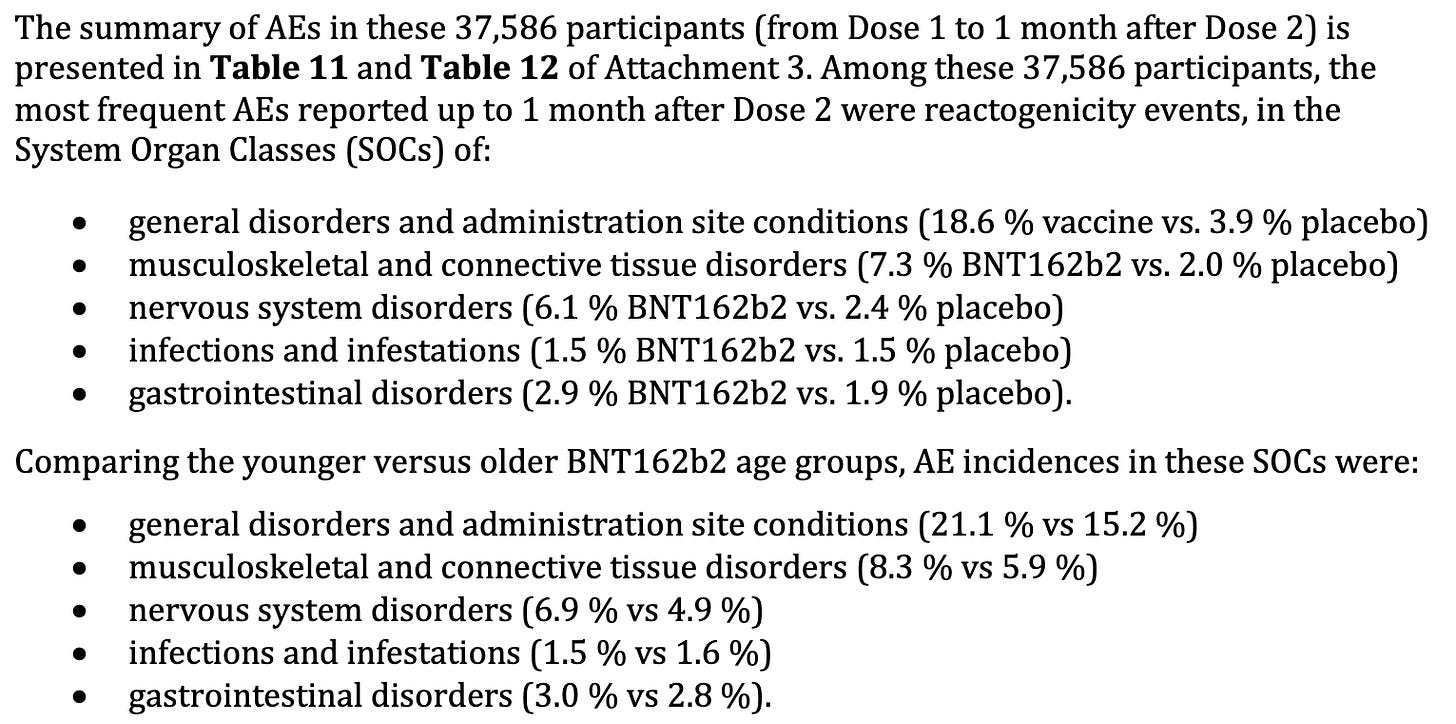
With regard to AEs, the rates of musculoskeletal and connective tissue disorders, nervous system disorders in the ‘drug arms’ and more than twice than placebo arms. This means that the products are causing increased rates of these disorders. General disorders (whatever that means), were reported to occur at 18% vs 3.9% in drug versus placebo arms. I would say that is worth mentioning to the public.
For example, if you inject 5 billion people with this stuff, that means that 930,000,000 will experience ‘General disorders’, 365,000,000 will experience musculoskeletal and connective tissue disorders, 305,000,000 will experience nervous system disorders, 75,000,000 will experience infections or infestations, 145,000,000 will experience gastrointestinal disorders. And don’t forget, this is only what has been reported for up to 2 months. There is the distinct possibility of delayed response adverse event occurrence.
Go to the Appendix on pages 26, 27 and 28.
These are the revised comments from the updated document being discussed as per this study. Notice, the previously written line: “Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity.
And it continues on the following page.
The Pregnancy Category is B2.
Category B2
Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed.
Studies in animals are inadequate or may be lacking, but available data show no evidence of an increased occurrence of fetal damage.
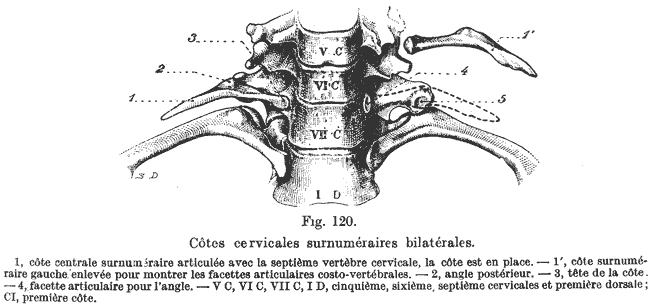
What is a supernumerary lumbar rib?1 It is a defect of development that results in extra ribs. This actually occurs in about 0.5% of the human population and can impose pain.
The readers should know that the second version of this document (which precedes this version), rates pregnancy in Category B1. This can be found here on page 7.
This means that they changed the rating from:
Studies in animals have not shown evidence of an increased occurrence of fetal damage
to
Studies in animals are inadequate or may be lacking but available data show no evidence of an increased occurrence of fetal damage.
Also, pertaining to preclinical safety data, they appear to have deleted the phrases that indicate a lack of hazard for humans and reversibility.
That’s all for now folks. Time to update VAERS data on website.
Chengetanai, S., Nchabeleng, E. K., Bacci, N., Billings, B. K., & Mazengenya, P. (2017). Supernumerary lumbar ribs: a rare occurrence on an adult African male skeleton. Anatomy & cell biology, 50(2), 155–158. https://doi.org/10.5115/acb.2017.50.2.155.












After reading this I was slightly nauseaous. How on earth did these toxic experimental biologicals ever manage to get approval for administration to humans. Thank you for all your work here. The findings are horrific and hard to digest.🥲🥲
Great Britain News. Booster shots are failing bad. Official government data shows 3x the cases and nearly 4x the hospitalizations and deaths in boostered.
Juicy bits starts at approximately 09:30
https://youtu.be/a8kdH2Xgf-k