Are the manufacturers using ionizable cationic lipids with esters in their LNP formulations?
The question that everyone wants to know the answer to!
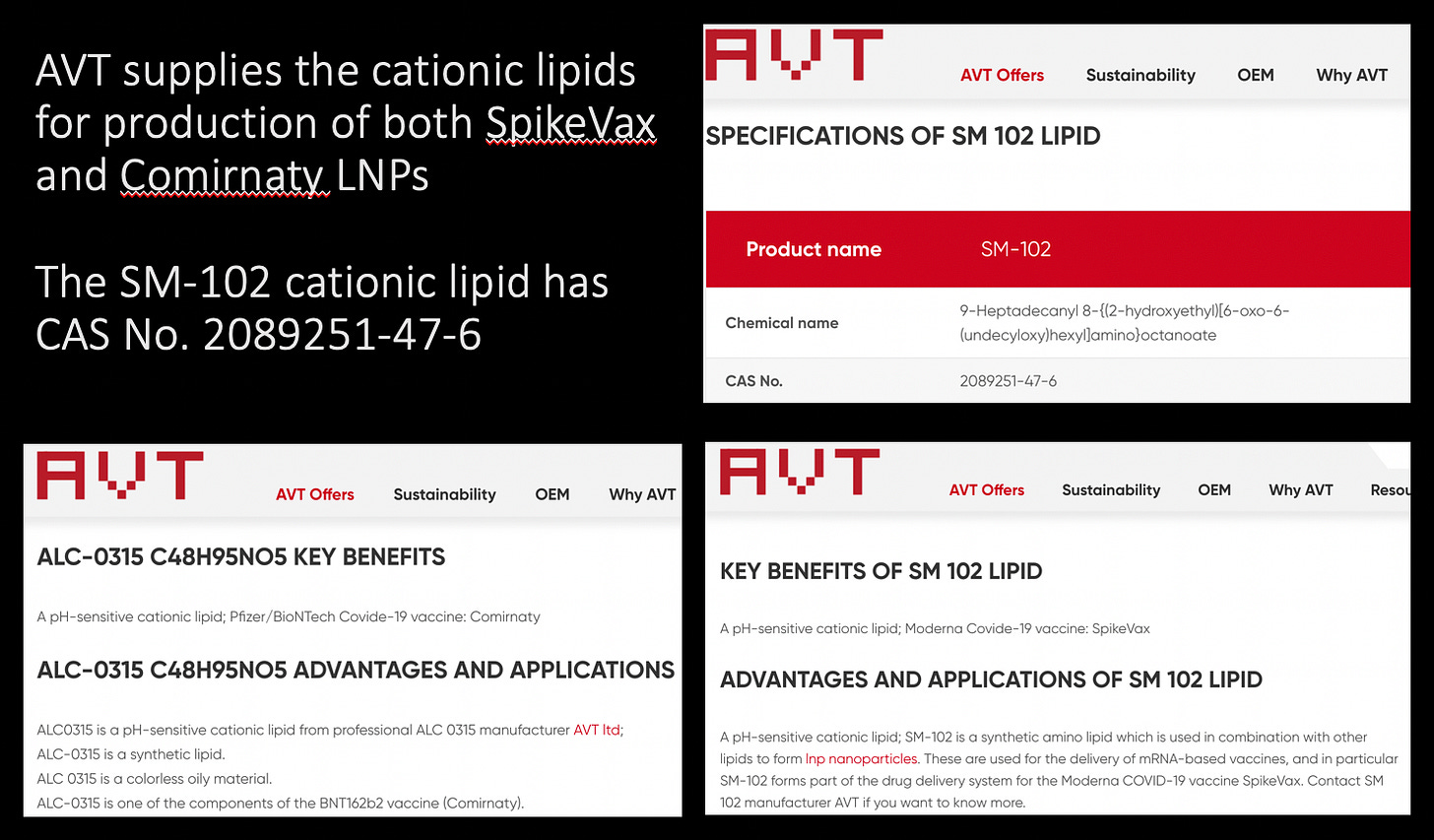
Please go to this supplier’s website. I found this yesterday and did some extra reading on the chemical names, CAS numbers and esters. AVT supply the SpikeVax and the Comirnaty cationic lipids.
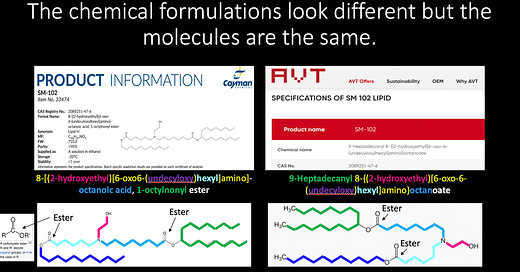
Let’s focus on SM-102. The CAS Registry No. is a unique identification number assigned by the Chemical Abstracts Service (CAS)1 that allows the identification of a specific chemical by number. The CAS No. for the SM-102 product supplied by AVT for use to produce LNPs for SpikeVax is 2089251-47-6. The Chemical name is horrific:
9-Heptadecanyl 8-{(2-hydroxyethyl)[6-oxo-6-(undecyloxy)hexyl]amino} octanoate.2
Below is the full product information for SM-102 from the Cayman Chemical company. The CAS Registry No. number for their SM-102 is 2089251-47-6. Perhaps they supply AVT? The ‘Formal name’ (Chemical name) looks different though:
8-[(2-hydroxyethyl)[6-oxo6-(undecyloxy)hexyl]amino]-octanoic acid, 1-octylnonyl ester.
There’s a video someone sent to me that you can watch here made by ‘Amazing Polly’ where she asks valid questions about the CAS for SM-102 and the differences in the chemical names.
I answer some of them.
Let’s put the Cayman and the AVT SM-102s side-by-each and compare their Chemical names.
It took me quite a while to make this gorgeous slide but it was so worth it. I actually secretly love chemistry. Don’t tell Ben. I color-coded all of the bits that link to the do-deca-bla-bla bits in their respective names. The 8- and the 6- in front of the oxo6s refer to the placements of the esters (yellow) 8 and 6 spots away from the amines (N). I think. I am not a chemist, remember. The esters are labelled.3 Of note, there are 2 esters per molecule shown. The colored sections in the molecules match, and there are no extra bits that I can see that would imply any difference between them.
Therefore, the molecules are in fact, the same, they just have their names written slightly differently. So the fact that they have the same CAS number makes sense. Mystère résolu.
I was actually relieved to find this out and the reason I was relieved is because I was able to confirm that the SM-102 product being supplied for the SpikeVax formulation by AVT has esters. Why is that important? Degradation issues associated with these molecules - that’s why.
Biodegradable lipids avoid lipid accumulation and untoward cytotoxicity because they are rapidly eliminated.4
I think this is a really important talking point considering the FOIA-requested bioaccumulation data from Japan.567 Is it possible that the ALC-0315 ionizable cationic lipids from their studies simply didn't degrade due to problems or inefficiencies with esters, and thus were shown to bioaccumulate in a number of organs, including the ovaries? Did they use ethers instead of esters? I honestly don't know.
Let’s go backwards a bit and talk about how cationic lipids went from being insanely toxic to just a ‘wee bit toxic’. Question: Did they?
Though cationic lipids found early success in delivering nucleic acids, their suitability for therapeutic use is compromised by safety risks.
Furthermore, cationic lipids are incompatible with negatively charged biological membranes, causing cell membrane disruption and resulting in unacceptable cytotoxicity.89
Unacceptable, eh? That sounds like something I can relate to. Badoom doom cha. Love to the UK crew.
Please refer to the Cayman News and Announcements site where they describe how to “[Tune] Ionizable Cationic Lipid Design for Efficacy and Safety”. The logic behind how they were able to supersede the problems of toxicity revolves around two main things: 1. the use the ionizable cationic lipids, and 2. the addition of esters to improve biodegradability.10 The first FDA-approved LNP formulation was called DLin-MC3-DMA (click this link and read the product description) and is the foundation upon which the SM-102 and the famous lipid 5.11121314 Is this like the fat version of the Jackson 5? How do they come up with these names? Lipid 5?! How very descriptive.
Let’s go through points 1 and 2.
On ionizable cationic lipids and bioaccumulation issues
Cationic lipids are positively-charged fat molecules that do not occur in nature. The ‘cation’ part means that they have fewer electrons than protons per ion, so their net electric charge is positive, as a result. With regard to cell membranes, this is bad news. Cationic lipids are exceedingly toxic to cells due to their charge properties. They literally rip membranes apart, if exposed.
Although the idea to use them in the context of constructing non-toxic liposomes/LNPs was a good one in theory, it didn’t work in reality - the reality being the clinical setting. Question: What if you could control ‘when’ the cationic lipids was positively-charged? Pieter Cullis could tell you the answer.15 Or I could try. Enter ionizable cationic lipids.
Ionizable cationic lipids are positively-charged only under certain conditions, like low pH found in endosomes. What if you could engineer the cationic lipids to be neutral in blood? And in the cytoplasm of cells? What if you could exploit the fact that certain fats like low-density lipoprotein (LDL) are released in endosomes because of the lower pH? This is precisely what Prof. Cullis did. He (and many talented lackies) designed multitudes of different ionizable cationic lipids that were positively-charged only at low pH.
They write:
At neutral pH (~7), ionizable cationic lipids have a near-zero charge, improving the stability and avoiding the cytotoxicity observed using LNPs formulated with cationic lipids.16
So, combine:
the ability to of these ionizable cationic lipids to integrate with the other fats that comprise the LNPs during micro-fluidic mixing
the ability to complex with negatively-charged nucleic acids (think mRNA), and
the formation of non-bilayer structures (due to positive re-charging from low pH) by interaction with negatively-charged lipids to enable endosomal escape and the subsequent release of their nucleic acid payload,
and you have a perfect solution! DEPLOY! It seems like a dream come true, doesn’t it? I heard once that if something sounds too good to be true after 20 years of trying when you need this dream to come true because someone is threatening your family if you don’t make it come true, then it probably is. Call me a sarcastic asshole skeptic. I really won’t mind. I am making no claims here with regard to people specifically referenced. Just being a sarcastic asshole.
Even though many successes were had (and don’t get me wrong, I respect the amazing advances in biochemistry), the first versions of the LNPs made using ionizable cationic lipids were still problematic in two main ways: the cationic lipids were still toxic, and the LNPs themselves bioaccumulated making them ‘untowardly cytotoxic’. What I have read on the problem-solving used to reduce toxicity of the ionizable cationic lipids in the clinical setting, does not convince me that the issues were adequately addressed in the context of use in living beings. Granted, it is hard to convince me of things that weren’t designed by nature. So I’m going to leave this point alone. Verdict: Not satisfied. Challenge me.
Giuseppe Ciaramella might simply explain away the problems by describing the defeat of the devil in the details, for example.
Onto degradation issues. They also came up with the idea to help overcome bioaccumulation of LNPs in vivo by making them more degradable by adding esters to the SM-102 molecules.
Here’s a neat video about esters and how they are linked within molecules.
When you add ester to a molecule you apparently are doing a condensation reaction and water is produced. Nice. So I guess the theory behind adding these to increase degradation is that esters undergo enzymatic hydrolysis in the cell and thus the lipid molecule degrades rapidly. Cool.
Alrighty, let’s get back to the title question: Are the manufacturers using ionizable cationic lipids with esters in their LNP formulations to result in fairly nontoxic and degradable LNPs that are safe for human use?
I would like to think so, but there’s no way to verify this without access to their ‘proprietary’ stuff. What I do know is that it is possible based on the biochemistry, in theory, to make an ‘effective’ (by effective, I mean a delivery vehicle for nucleic acid stuff) LNP with minor toxicity. In theory. Whether or not these LNPs exhibit reduced toxicity in the living being, remains to be seen in my eyes. As pointed out by Thomas Madden - supreme leader of Acuitas Therapeutics - “You can have 50 different ionizable lipids that all deliver effectively to cells in culture, and 49 of them won’t work a damn in vivo.”17 I agree with Thomas and I am not a gambler. Are the manufacturers? In light of the rushed, emergency, chaos, operation warp speed, psychotic billionaire, pharmaceutical giant, lack of regulation thing that happened, I once again, am skeptical. Prove me wrong. There's also all the data that I've been presenting for over 2 years. There's that.
Summary
LNPs embedded with whatever (including modified mRNA) can be produced using high tech buzzing shiny micro-fluidic mixers. Concentrations of each of the lipids can be tweaked to make half-decently consistent LNPs that are presumably not that toxic. The ionizable cationic lipids themselves aren’t really cationic lipids when introduced to the cells by endocytosis, they rather ‘become’ cationic lipids when exposed to low pH such as is the case in an endosome. This reaction presumably induces LNP alteration and release of the modified mRNA, in the context of the COVID-19 injectable products. But, by my assessment, the exact mechanism by which this release occurs is, as yet, not clearly defined. The other thing that nags me is the real ability to up-scale LNP (mRNA encapsulation) production such that the LNPs are of uniform size because apparently, this really matters. More on this soon…
Every time I ask a question, I learn a bunch but end up with more questions and no solid answers. I welcome the chiming in of you brilliant crew and if there are any chemists out there who want to add to this piece, PLEASE DO! :)
https://en.wikipedia.org/wiki/CAS_Registry_Number
https://en.wikipedia.org//wiki/SM-102
https://en.wikipedia.org/wiki/Ester
Hou, X., Zaks, T., Langer, R., et al. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6(12), 1078-1094 (2021).
Pfizer/Comirnaty- 125742_S1_M2_24_nonclinical-overview.pdf/125742_S1_M2_26_pharmkin-written/tabulated-summary.pdf; Jan; Feb 2021.
JAPANESE FOIA-requested study (Many thanks to Byram Bridle)
https://viralimmunologist.substack.com/p/inside-a-court-case?utm_source=post-email-title&publication_id=592214&post_id=96153963&isFreemail=true&utm_medium=email
Schlich, M., Palomba, R., Costabile, G., et al. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 6(2), e10213 (2021).
Zhang, Y., Dahal, U., Feng, Z.V., et al. Influence of surface ligand molecular structure on phospholipid membrane disruption by cationic nanoparticles. Langmuir 37(24), 7600-7610 (2021).
Zhi, D., Bai, Y., Yang, J., et al. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 253, 117-140 (2018).
https://www.caymanchem.com/news/tuning-ionizable-cationic-lipid-design-for-efficacy-and-safety
Maier, M.A., Jayaraman, M., Matsuda, S., et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21(8), 1570-1578 (2013).
Hassett, K.J., Benenato, K.E., Jacquinet, E., et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1-11 (2019).
Sabnis, S., Kumarasinghe, E.S., Salerno, T., et al. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26(6), 1509-1519 (2018).
https://en.wikipedia.org/wiki/Pieter_Cullis
Hou, X., Zaks, T., Langer, R., et al. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6(12), 1078-1094 (2021).
https://cen.acs.org/pharmaceuticals/drug-delivery/Without-lipid-shells-mRNA-vaccines/99/i8





I will not stop digging. And whatever I find I will blab to everyone who wants to hear.
Ionic trapping and even concentration is a well known trick to steer agents in terms of ADME & distribution. IIRC, nicotine accumulates in your stomach regardless of whether you smoke it, chew it or absorb it through the skin. It becomes charged at stomach pH.