The thrombotic bus: Are red blood cells agglutinating in injected people because of zeta potential disruption by spike?
Is PEG in the COVID shots aiding and abetting agglutination?
Before I start, I would advise a quick read of two papers.
A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody2
The first paper’s findings (albeit in 1973 and in a specific test system) show that zeta potential is not of great importance in increased agglutinability (is this actually a word?) of test cells.
The second paper finds that the SARS-CoV-2 spike protein binds red blood cells to induce clumping. What I want to know is, what would happen if SARS-2 expressed hemagglutinin esterase? Would we see this thrombotic nightmare in certain individuals who’ve been injected with the COVID-19 products?3 I got way ahead of myself there.
I’ve already penned a couple articles (you can read those here and here) about the effects of spike binding RBD-associated CD147 that leads to the demise of red blood cells (RBCs) by hemolysis, so this article is about another thing that can happen when you mix spike with RBCs that doesn’t involve binding of receptors, but direct ‘adhesion’ of spike and RBCs via the RBD and sialic acid, respectively. Again, I got way ahead of myself here.
Background
On RBC agglutination (hemagglutination)
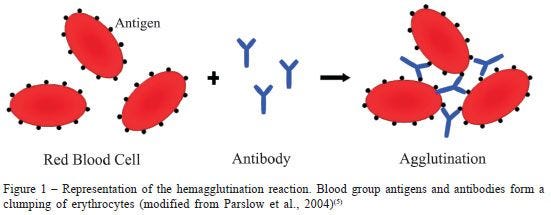
The word agglutination has its origins in the 1540s from the Latin agglutinationem, "act of uniting by glue."4 Heme refers to blood from the Latin haemo.5 Thus hemagglutination is blood gluing basically. The glue, in the case of RBC agglutination, is the antibody, and the electrical environment permitting this agglutination in the first place as shown in Figure 1.6
In the world of ‘disease’, these antibodies can be produced when a very unlucky person is exposed to cold temperatures. These particular antibodies are called ‘cold agglutinins’. This is a very rare ‘disease’, by the way. Agglutinins also play a role in some viral infections78 such as Epstein-Barr Virus, in bacterial infections9 such as Mycoplasma pneumoniae, or from lymphoproliferative disorders.
If you’ve ever wondered why people with different blood types can’t share blood randomly, it’s because of hemagglutination - antibodies to A type will bind cells with B blood group antigens. You can read a bit about that here.
As a point of interest because we love viruses (speak for yourself Jess), hemagglutinins are proteins on the surfaces of viruses in the Paramyxoviridae family that induce hemagglutination. An example of such a virus is the Influenza virus. The ‘H’ in ‘H1N1’ virus, for example, stands for Hemagglutinin and the number refers to the type of hemagglutinin. The ‘N’ stands for Neuraminidase and it helps with the infection process.
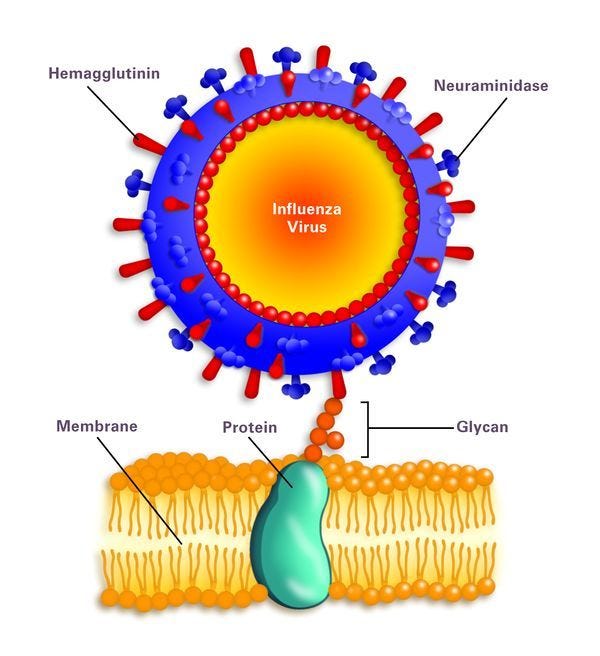
So, agglutinins, in general, are proteins that induce aggregate formation in the blood.
I know what you’re thinking. Does SARS-CoV-2 induce hemagglutination? I will get back to this.10
On colloidal mixtures
We can’t talk about zeta potential without first understanding what colloids are. Colloids are mixtures: suspensions of insoluble particles dispersed throughout another substance of a different state. When I say ‘state’, I am referring to either a solid, a liquid or a gas. For example, whipped cream is a gas-liquid colloid and blood is a solid-liquid colloid called a sol. Particles in colloids exhibit Brownian motion which is kind of like when you walk home drunk after a fun night out.
Colloids can be mixtures of liquids, solids and gases. Colloids are defined by particle size (1 nm and 1000 nm): they are large enough to scatter light. This is called the Tyndall Effect. Below is a chart that shows various examples of colloids with the respective names. For example, those marshmallows that we all toast on a stick over a campfire are colloids called solid foam. They are actually gases in a solid medium! Colloids can also be unstable (non-homogeneous dispersion of particles) or stable (homogenous dispersion of particles) and this will depend on the zeta potentials of the particles.
On zeta potential
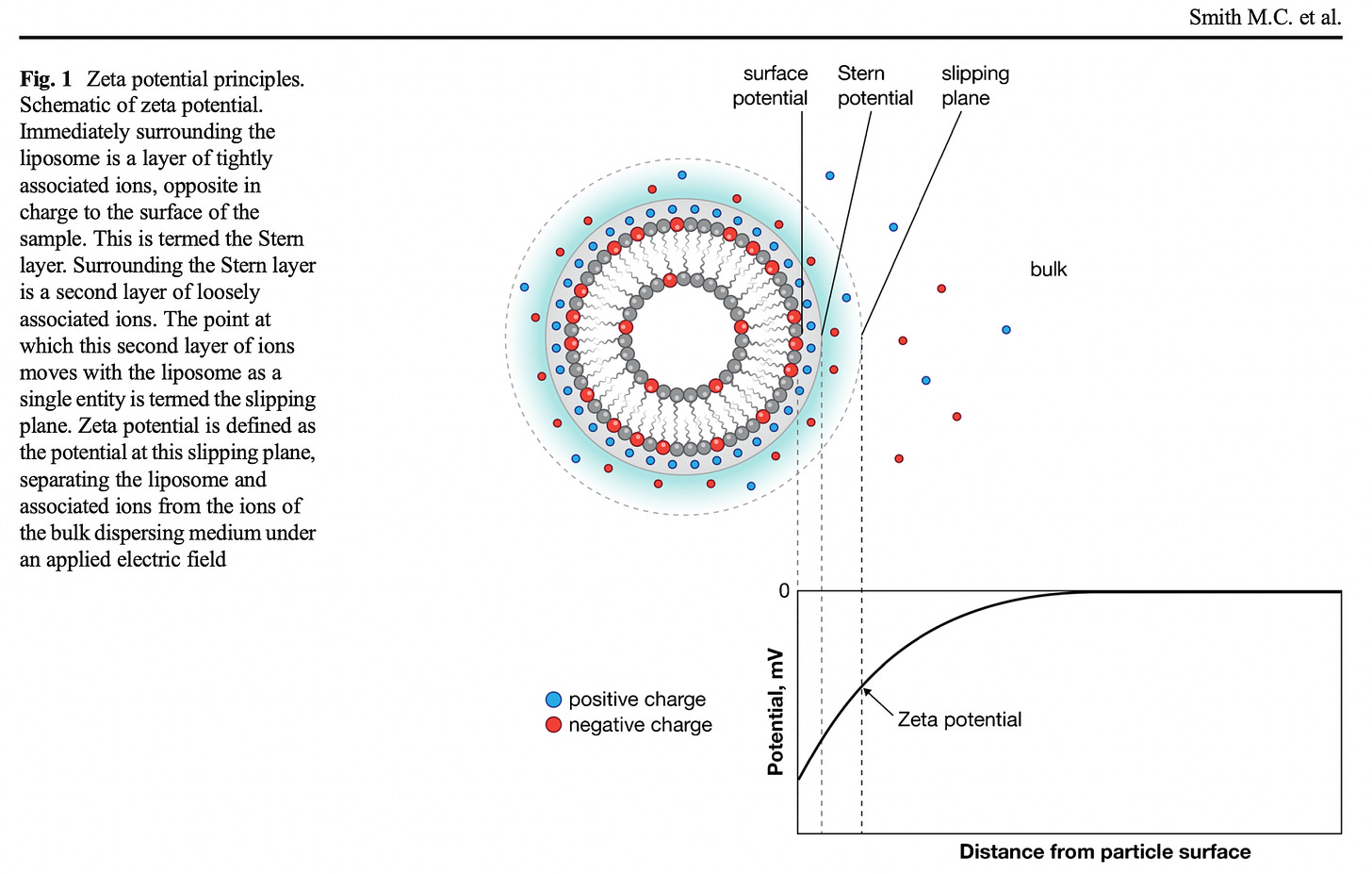
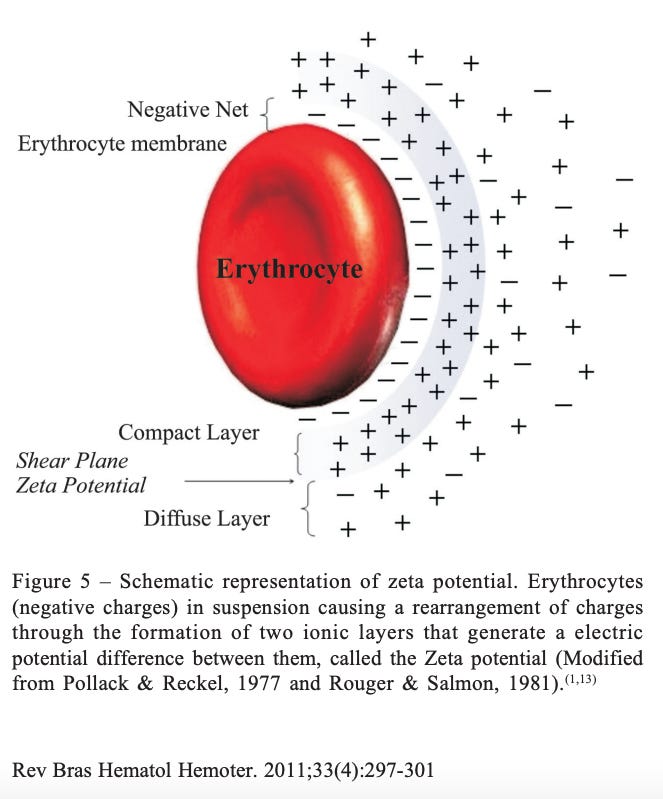
The reason why colloids are colloids is because of the repulsive forces between particles in a mixture (note: cationic properties of medium if particles are negatively charged). For example, negatively-charged particles in a mixture will repel each other, and this is precisely due to zeta potential. Negatively-charged particles are layered by a tight sphere of positive ions which is called the Stern layer, and another looser sphere of positive and negative ions called the double layer, as shown in figure 5. The boundary between the double layer and the ‘outside’ of the particle is called the ‘Slipping plane’ and the zeta potential is the voltage at the edge of the Slipping plane with respect to medium where the particle’s surface does not associate with ions, molecules or other things.
It is effectively the physical property exhibited by any colloidal system: the differential between the Stern layer and the double layer that manifests as repulsion. In other words, RBCs don’t stick together in the blood because they are all the same charge and they repel each other due to high zeta potential.11
If two adjacent particles have sufficiently high zeta potential of the same sign, they will not agglomerate due to repulsive electrostatic forces between particles with like charges.12
So, if you think about it, if you lower these high zeta potential, the particles likely will agglomerate. What if those particles are red blood cells?
On zeta potential of RBCs
Zeta potential is defined as the degree of negative charge on the surface of a red blood cell; it is the potential difference between the negative charges on the RBCs and the cations in the fluid portion of the blood.13
RBCs have a negative charge on their surfaces due to sialic acid.141516 Sialic acids are terminal (at the end), negatively charged monosaccharides (simple sugars) present on many N- and O-glycans (sugar chains).17 SARS-CoV-2, and all coronaviruses, love sialic acid, by the way, and readily attach to cells with these sialic-tipped glycans via spike protein. (See reference #2) (That might be the most important phrase in this article, and that anyone could write, that I threw in here as a quasi-'side-note'.) Imagine the horror show already: SARS-CoV-2 spike binds RBCs irrespective of ACE-2 or CD147 and clumps them together. Think about it.
SARS-CoV-2 binds to RBCs in vitro and also in the blood of COVID-19 patients; (2) although ACE2 is its target for viral fusion and replication, SARS-CoV-2 initially attaches to sialic acid (SA) terminal moieties on host cell membranes via glycans on its spike protein… (See reference #2).

So besides the fact that we already know that SARS-CoV-2 spike binds RBCs and clumps them together, and that therefore, the zeta potentials between the RBCs are 'overcome' (the aggregation force is greater than the force of repulsion), what do we know about other factors that can aid and abet the overcoming of the repulsive forces and the reduction of zeta potentials in the first place?
The zeta potential of RBCs can be affected by pH, ionic strength, osmotic pressure and temperature.18 Small changes in any of these parameters can potentially have dramatic effects on the zeta potential values.19 Below is schematic showing the zeta potential of an RBC (erythrocyte).
The magnitude of the zeta potential depends on the net charge density of surrounding cations, that is, on the ionic strength. Thus, a decrease in ionic strength will result in a reduction in the thickness of the double layer due to increased cations density resulting in the need to maintain electrical neutrality. This further reduces the zeta potential. (See reference #6)
Interjection: Look at the following schematics and think about zeta potential in the context of direct hemagglutination by a virus that induces hemagglutination. The antibodies that normally glue the RBCs can be replaced by viruses (or viral bits) themselves in some cases. In the schematic on the right of Figure 8, it is shown that the gap between 2 RBCs that is bridged by an IgG or IgM antibody is 79 angstroms (Å). For perspective, that is 7.9 nanometers (nm). The SARS-CoV virus is ~100 nm on average and the spike protein alone is ~10 nm (length)20. An IgG antibody is ~13.7 nm wide21 so it seems, at least size-wise, that it's a good fit for the spike protein in terms of bindability. What about the spike's charge? The RBD of the spike protein is positively charged and more positively charged according to variant, ie: Omicron's RBD is more strongly positive with regard to net electrostatic surface charge, relative to the original chicken Wuhan recipe RBD.22
It seems really easy to imagine that the top end of spike protein that houses the RBD can bind a couple of RBCs.
What interviral/interspike-RBC forces would have to be overcome in order for spike to bind those sialic acids? Hydrophobic bonds, Van der Walls and electrostatic forces and hydrogen bonds all come to mind, in addition to repulsive electric forces such as the zeta potential. That’s a lot of forces!
Keep these forces in mind and let’s return to pH, ionic strength, osmotic pressure and temperature, which are all inherently intertwined with the above mentioned forces and bonds. Assuming that one of these parameters underwent a small change, then potentially, one of the effects on the zeta potential values of the RBCs, would be their reduction. If the zeta potential was reduced, then the RBCs could aggregate. In my research, I found four things that can reduce the zeta potential of RBCs by decreasing the ionic strength of the medium:
Albumin
LISS (Low ionic strength solution)
Proteolytic Enzyme
PEG (Polyethylene Glycol) (See reference #6)
Physiochemical properties such as particle size and zeta potentials can be simply manipulated and controlled by varying the key processing parameters such as CS, PEG concentration and CS/TPP mass ratio.23
PEG is one of four constituent lipids in the lipid nanoparticle formulations used by Moderna and Pfizer for the delivery of mRNA in the COVID-19 injectable products. “Polyethylene Glycol causes dehydration of cells and potentiates agglutination.”24 The way in which PEG potentiates agglutination of RBCs (I think) is by removing water molecules from the sialic acids to free up the sites for hemagglutinin binding, subsequently improving agglutination ability. (I might have this completely wrong, but from what I understand, this is the mechanism of action.) The net effect will be a reduction in electrostatic potential.
The final diluted injectable dose of 0.3 ml of the COVID-19 injectable products contains the following ingredients listed in Figure 10, including 30 mcg of a nucleoside modified messenger RNA (modRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2 and 0.05 mg 2[(polyethylene glycol)-2000]-N,N-ditetradecyclacetamide.

Is the 0.05 mg PEG in a 0.3 ml Pfizer injection enough to lower RBC zeta potential and potentially induce agglutination of RBCs?25 We can do some calculating to make an educated guess as to the ratio of PEG molecules per RBC, and then determine if this ratio is enough to disrupt the zeta potential of the RBC to potentiate agglutination. (See Figure 11 for my calculations.) Assuming homogenous distribution of PEG molecules in the blood, we get a ratio of 1,572:1 PEG molecules to RBCs since there are 5,000,000 RBCs in 1 cubic millimeter of blood.26 Assuming a concentration of molecules at the injection site, for example, this ratio would be even higher.
I haven't located a paper that confirms the ratio of PEG:RBCs that can lower the zeta potential of RBCs to induce hemagglutination yet - I will update the article when I find it. Reference #19 paper is good, but reports changes to zeta potentials of the LNPs themselves, not RBCs. For now, my gut tells me this is enough PEG to disrupt the zeta potential of RBCs. This idea also lends itself to the hypothesis that aspiration during injection is a determinant of adverse event frequency. The reason I say this is because if there is, in fact, enough PEG in the blood - assuming no aspiration and subsequent injection of product into the blood stream via a vessel - to lower the zeta potential of RBCs to cause them to clump, then we would only see a fraction of injected people suffering agglutination of their RBCs, ie: the fraction that got this stuff injected directly to the blood. And this is what we are seeing. I would also imagine that repeated doses of the PEG-coated LNP capsules would probably exacerbate any RBC agglutination that had already ensued.
What effects do the other ingredients (there are a lot of salts, for example) have on the ionic strength in the blood medium in general? I would say, negligible effects, and certainly less than those determined by simple daily food and water ingestion. Food and water have far greater effects on ionic strength of blood - more than an injection with salts ever could, in my opinion. I mean, unless there was something really insidious in the injection, like a host-assisted/self-amplifying protein that glued RBCs together.
Back to the title question
Are red blood cells agglutinating in injected people because of zeta potential disruption?
Zeta potential disruption would need to occur in order for the spike to bind sialic-acid tipped glycans on RBCs in the first place, so again, the more pertinent question is: are other factors, like PEG, involved that potentiate agglutination by lowering the zeta potential of RBCs? I believe quantitative experiments to determine the effects of various PEG, salt and sugar concentrations and temperature changes for example, need to be done to define this physicochemical binding. It is possible that people suffering the worst thrombotic effects following injection simply have 'gummier' blood to begin with based on the ionic strength of their blood (alkaline water can also help to lower blood viscosity).27
There are so many questions that need answering. Thrombotic adverse events associated with these shots are off the charts (N = 51,600 w/URF -> 1,599,600; total shot administration number: 673,181,22028 = 1/420 people). Agglutination of RBCs, equating to sticky or clumpy blood, seems to be a much more prevalent clinical pathology since 2021. I can’t imagine why. It is highly likely, that the blood-clotting-related adverse events being reported to every pharmacovigilance database in the world could be explained by spike-mediated hemagglutination (and apparently, curcumin prevents it29).
The combined effects of the highly (N-)glycosylated spike protein30 acting as RBC fly tape, and the changes in ionic charge or balance of the blood due to potential changes in blood chemistry induced by a multitude of factors like electrolyte levels, could certainly enhance hemagglutination. Add PEG to the mix, and this might exacerbate hemagglutination by lowering RBC zeta potential.
P.S. Cationic lipids have high zeta potentials. (See reference #19.) So I wonder what effect the introduction of lipids with high zeta potentials has on RBCs?
Oh, and I don’t mean to mess with your minds, but magnets also affect zeta potential. You can read about that in an article entitled: "Magnetic Effects on Zeta Potential and Diffusivity of Nonmagnetic Colloidal Particles".31
In addition to an alkaline diet and daily turmeric, try grounding every day as part of a healthy lifestyle - that includes free flowing non-sticky blood. :)
Stratton, F., Rawlinson, V. I., Gunson, H. H., & Phillips, P. K. (1973). The Role of Zeta Potential in Rh Agglutination. Vox Sanguinis, 24(3), 273–279. doi:10.1111/j.1423-0410.1973.tb02641.x
Scheim, D.E. A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody. Int. J. Mol. Sci. 2022, 23, 2558. https:// doi.org/10.3390/ijms23052558.
Zeng, Q., Langereis, M. A., van Vliet, A. L. W., Huizinga, E. G., & de Groot, R. J. (2008). Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proceedings of the National Academy of Sciences, 105(26), 9065–9069. doi:10.1073/pnas.0800502105.
https://www.etymonline.com/search?q=agglutination
https://www.etymonline.com/word/hemo-?ref=etymonline_crossreference
Fernandes HP, Cesar CL, Barjas-Castro Mde L. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev Bras Hematol Hemoter. 2011;33(4):297-301. doi: 10.5581/1516-8484.20110080. PMID: 23049321; PMCID: PMC3415751.
Briody B.A. Jr. Hemagglutination induced by viruses. Yale J Biol Med. 1946 Oct;19(1):29-61. PMID: 20275721; PMCID: PMC2602042.
Shida H. Vaccinia virus hemagglutinin. Subcell Biochem. 1989;15:405-40. doi: 10.1007/978-1-4899-1675-4_12. PMID: 2678619.
Ishikawa Y, Urano-Tashiro Y, Yamanaka Y, Saiki K, Hayashida N, Takahashi Y. Hemagglutinating properties of a Streptococcus gordonii strain expressing sialic acid-binding adhesin homolog with low binding site similarity to that of strain DL1. J Oral Biosci. 2022 Jun;64(2):253-258. doi: 10.1016/j.job.2022.03.001. Epub 2022 Mar 11. PMID: 35288286.
Manzanares-Meza LD, Medina-Contreras O. SARS-CoV-2 and influenza: a comparative overview and treatment implications. Bol Med Hosp Infant Mex. 2020;77(5):262-273. English. doi: 10.24875/BMHIM.20000183. PMID: 33064680.
https://www.research.colostate.edu/wp-content/uploads/2018/11/ZetaPotential-Introduction-in-30min-Malvern.pdf
https://nanocomposix.com/pages/zeta-potential-measurements
https://www.labce.com/spg813211_zeta_potential_and_van_der_waals_forces.aspx
https://medicallabtechnology.com/negative-zeta-potential-red-blood-cells/
Durocher JR, Payne RC, Conrad ME. Role of sialic acid in erythrocyte survival. Blood. 1975 Jan;45(1):11-20. PMID: 803103.
Kahane, I., Polliack, A., Rachmilewitz, E. et al. Distribution of sialic acids on the red blood cell membrane in β thalassaemia. Nature 271, 674–675 (1978). https://doi.org/10.1038/271674a0.
https://www.ludger.com/glycan-analysis-services/sialic-acid-analysis.php
Sv. Jovtchev, N. Hristova, S. Stoeff, T. Galabova & S. Stoylov (2009) Investigations on the Polymer Induced Aggregation of Red Blood Cells, Biotechnology & Biotechnological Equipment, 23:sup1, 581-584, DOI: 10.1080/13102818.2009.10818492.
Smith, M. C., Crist, R. M., Clogston, J. D., & McNeil, S. E. (2017). Zeta potential: a case study of cationic, anionic, and neutral liposomes. Analytical and Bioanalytical Chemistry, 409(24), 5779–5787. doi:10.1007/s00216-017-0527-z.
Yinon M Bar-On, Avi Flamholz, Rob Phillips, Ron Milo. (2020). Science Forum: SARS-CoV-2 (COVID-19) by the numbers. eLife 9:e57309.
Tan, Y. H., Liu, M., Nolting, B., Go, J. G., Gervay-Hague, J., & Liu, G. (2008). A Nanoengineering Approach for Investigation and Regulation of Protein Immobilization. ACS Nano, 2(11), 2374–2384. doi:10.1021/nn800508f.
Gan Hin Hark, Zinno John, Piano Fabio, Gunsalus Kristin C. Omicron Spike Protein Has a Positive Electrostatic Surface That Promotes ACE2 Recognition and Antibody Escape. Frontiers in Virology. Volume 2, 2022. 10.3389/fviro.2022.894531.
Shah, Sunil & Pal, Angshuman & Kaushik, V. & Devi, Surekha. (2009). Preparation and Characterization of Venlafaxine Hydrochloride-Loaded Chitosan Nanoparticles and In Vitro Release of Drug. Journal of Applied Polymer Science. 112. 2876 - 2887. 10.1002/app.29807.
https://medicallabtechnology.com/negative-zeta-potential-red-blood-cells/
https://www.fda.gov/media/144412/download
Sharma R, Sharma S. Physiology, Blood Volume. 2022 Apr 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 30252333.
https://premiumwaters.com/blog/alkaline-electrolyte-water-difference/
https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-booster-percent-pop5
https://professional.diabetes.org/abstract/curcumin-prevents-glycosylation-proteins-and-oxidative-stress-human-red-blood-cells-rbc
Sanda M, Morrison L, Goldman R. N- and O-Glycosylation of the SARS-CoV-2 Spike Protein. Anal Chem. 2021 Feb 2;93(4):2003-2009. doi: 10.1021/acs.analchem.0c03173. Epub 2021 Jan 6. PMID: 33406838; PMCID: PMC7805595.
Higashitani, K., Iseri, H., Okuhara, K., Kage, A., & Hatade, S. (1995). Magnetic Effects on Zeta Potential and Diffusivity of Nonmagnetic Colloidal Particles. Journal of Colloid and Interface Science, 172(2), 383–388. doi:10.1006/jcis.1995.1268.







Related info: "Inhibition of spike protein-induced HA was tested using the macrocyclic lactone ivermectin (IVM), which is indicated to bind strongly to SARS-CoV-2 spike protein glycan sites. The results of these experiments were, first, that spike protein from these four lineages of SARS-CoV-2 induced HA. Omicron induced HA at a significantly lower threshold concentration of spike protein than for the three prior lineages and was much more electropositive on its central spike protein region. IVM blocked HA when added to RBCs prior to spike protein and reversed HA when added afterwards. These results validate and extend prior findings on the role of glycan bindings of viral spike protein in COVID-19." (Boschi, et al, 2022, preprint) SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects, https://www.biorxiv.org/content/10.1101/2022.11.24.517882v1
Love the analogies they are super helpful.. yesterday Del Bigtree & Ryan Cole took a tour of the lab and did slides with Dell's blood and jab contents. The reaction of the blood to one drop of Pfizer looks very much like what you describe here. Starts @ 60 min https://thehighwire.com/watch/