A summary of Kevin McKernan's latest deep dive... there's likely also a dsRNA problem with the modified mRNA LNP platform
Thanks to Maria Gutschi, Maarten Fornerod and Janci Lindsay for having inquiring minds. And everyone else.
Here’s the original article.
Bottom line for this summary and add-on article:
what’s the deal with double-stranded RNA (dsRNA) in the modified mRNA injectable products?
why is this a big deal?
can something be done?
So everybody here probably knows that we’re on the verge of publishing our latest results on the DNA contamination in found in the Pfizer and Moderna lots. We’ve updated the reports originating from VAERS for the vials tested, and are slowly filling in what we believe is a dose-response curve: SAE/total AE against DNA measured by qPCR for spike and plasmid ORI.
But there are so many questions that keep popping up along the way in our investigation. Maria Gutschi wondered: “What of dsRNA?” If dsRNA is linked to myocarditis, and there is more dsRNA in the Moderna products, then maybe this explains the affinity for reports of myocarditis in the Moderna context?
So Kevin did due diligence (his poor projects - which are what he ACTUALLY does for a living - must stand aside again for now: the cost of being an actual scientist), and checked the effect of something called RNaseIII on the modified mRNA products. He also threw in - very importantly - a viroid called Hop Latent Viroid (HpLVd) as a control. He did this very much on purpose because he knew that this viroid, which is an ssRNA molecule with ‘bubbles’, would not be chewed up by the RNaseIII.
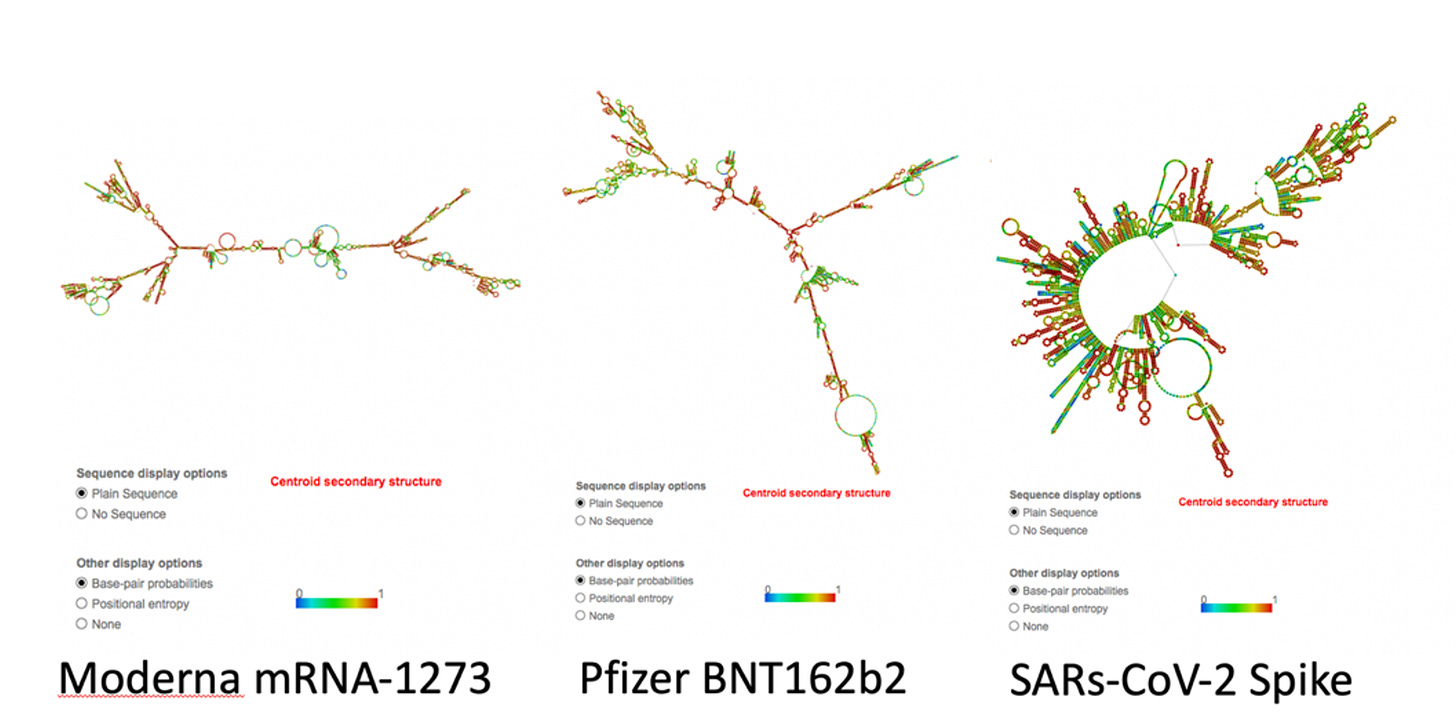

The Pfizer and Moderna RNAfold guys look a lot like viroids, don’t they? Complete with bubbles. Well, guess what? The dsRNA-binding antibody used in ELISA detection methods, perhaps the very ones used in the context of the modified mRNA injectable products (to check for dsRNA presence), don’t bind to viroids. If they don’t bind to viroids based on their bubbly structure, and the modified mRNA is akin to a viroid based on structure, then perhaps the detection antibody doesn’t bind to the dsRNA in the modified mRNA products? Thanks to Janci Lindsay for papers on the detection methods. Good will hunting!
Back to RNaseIII. RNaseIII chews up dsRNA (cleaves it at specific targeted locations) and therefore, if there is dsRNA in the Pfizer or Moderna products, this enzyme will tell the tale of its presence. It should also not chew up our control viroid. Below is a chart that Kevin produced from a modified mRNA ribonuclease sensitivity assay. The methods can be found here. “RNA Broad Range Dye is selective for ssRNA over dsDNA.”

A keen observer will note that the RNaseIII buffer reduces the fluorometry read out for ModRNA (ng/ul) to a great extent. It’s a bit weird, but Kevin says the reduced signal can be explained by the presence of metals like Manganese. He’s checking on that. The repeat read duplicates this finding. So think of that as the baseline. The effect of the RNaseIII subsequent to ‘buffering’, reduces the signal even more. So go back to the post boil levels of ModRNA. The differences between these measurements and the post RNaseIII measurements are ‘yuge. This indicates that there is a massive amount of dsRNA in the vials.
So how is it possible that the regulators or manufacturers didn’t pick up on this? Why don’t they use RNaseIII prior to their measuring the amount of dsRNA in their products? Maybe they did. Maybe they saw this result and shat their pants?
Think back to the ELISA that they might be using. If these dsRNAs are like viroids, then the antibody wouldn’t bind them. No detection. This would be a very sneaky way to avoid detecting dsRNAs whilst trying to ‘clean them up’. The T7 polymerase can promote dsRNA production, and there are attempts being made to resolve this problem due to its known association with enhancing immunostimulatory effects. You can read about that here. So it seems like it’s known in the scientific community that the potential for dsRNA production during IVT exists, and this doesn’t even call into the added effect of the N1-metylpseudoUs.
So all in all, not only do we have DNA, we have dsRNA to deal with. This can lead to all sorts of problems from activation of cGAS-STING pathway to induce cancer in the case of cytosolic DNA, and “excessive or uncontrolled dsRNA immunostimulation can lead to autoimmune diseases, such as Aicardi-Goutières syndrome, and can also contribute to the development of chronic inflammatory diseases” in the case of dsRNA.
One more thing. And this is serious.
Aicardi-Goutières syndrome (AGS) is a rare genetic disorder that affects the brain, spinal cord, and immune system.
And guess what?
AGS is an inherited disorder caused by mutations in the TREX1, RNASEH2A, RNASEH2B, or RNASEH2C genes.1
TREX1. Well I’ll be damned. TREX1 is highly important in the cCAS-STING pathway.2 You can read about that here.
I tend to be someone who is skeptical of everything and here’s the thing about AGS as an inherited genetic disorder that makes me go hmm. Although there is currently no evidence that AGS can develop de novo from epigenetic modifications, it is possible that epigenetic changes may play a role in the progression or severity of AGS, since the IFN response can be influenced by epigenetic factors. And “no evidence” could mean a whole lot of things these days, couldn’t it. Maybe there’s just no evidence yet. Remember Galileo?
Here’s something that should shock you. There are 13 reports of Aicardi-Goutières in VAERS in the context of the COVID-19 injections, and 12 of them are listed with no prior history - that is: no underlying condition is reported. Meaning: de novo onset. There is 1 report filed with Aicardi-Goutières listed as an AE in VAERS prior to 2021 since 1990 for all vaccines combined that involve no history. This report was filed in in 2010 (and again from this person in 2019).
It’s possible that that the shots could be charging up an underlying condition, even though there is no history listed in these particular reports in VAERS.
Please look up VAERS_IDs: 394081 and 807888. It’s very, very interesting. These two reports are from the same person since the SYMPTOM_TEXT in each report refers to a court case related to MMR vaccines with many matching markers.
Vaccine court special master in a decision released April 27 rejected the government's contention that a fetus allegedly injured by a vaccine administered to the mother. VAERS_ID: 394081
Here’s the fully SYMPTOM_TEXT from VAERS_ID: 394081
This case was reported in a published article, regarding a Vaccine court special master in a decision released April 27 rejected the government's contention that a fetus allegedly injured by a vaccine administered to the mother had not "received" that vaccine under the terms of the act. The special master ruled in 2001 that the patient who suffered from Aicardi's syndrome, could not be said to have "received" the MMR vaccine in utero based on the most restrictive plausible meaning of the word as required at the time by the act's waiver of sovereign immunity. The special master said that he held the case on abeyance in the event that Congress addressed the issue, but instead, he said, the petitioners moved for reconsideration based on an intervening Court decision in physician Security Service. Under physician, the special master said, he may rule for the more persuasive definition, rather than the most limiting. Given that the act was a "remedial" statute, under physician, he said the more persuasive interpretation that the developing fetus receives a vaccine given in the mother event though it had neither ingested not been injected with it as described in the act. It was reported that it was concluded in 2001, that the statutory language was ambiguous in its application to this situation, and that both competing interpretations were at least "plausible" by the special master. Therefore, he concluded, that he was not free to choose the interpretation that he found to be more persuasive. He was bound under the 1990s cases rather, to choose the interpretation that would produce the most narrow and restricted waiver of sovereign immunity. In light on the physician opinion, however, the special master stated that he was free to weigh relative merits of the two competing interpretations. Further, physician specified that he was free to consider general principles of statutory construction other than the sovereign immunity canon of narrow construction, he could take into account the statutory consideration of liberal construction of remedial statutes. The special master directed the patient's parents to determine whether they could obtain an expert report that the vaccine likely progressed through the patient's mother body into the fetus she was carrying and, if so, whether it caused the patient's seizure disorder, brain malformation and significant developmental delay. The representing parent patient's information was reported. Additional information is not expected.
Here’s the full SYMPTOM_TEXT from VAERS_ID: 807888
This case was reported in a published article. It was ruled in 2001 that the patient who suffered from Aicardi's syndrome, could not be said to have "received" the measles virus vaccine live (Enders-Edmonston) (+) mumps virus vaccine live (Jeryl Lynn) (+) rubella virus vaccine live (Wistar RA 27/3) (HSA) vaccine in utero based on the most restrictive plausible meaning of the word as required at the time by the Vaccine Act's waiver of sovereign immunity. The judge said that he held the case on abeyance in the event that agency addressed the issue, but instead, he said, the petitioners moved for reconsideration based on an intervening court decision. Under decision, the judge said, he may rule for the more persuasive definition, rather than the most limiting. Given that the Vaccine Act was a "remedial" statute under the decision, he said the more persuasive interpretation that the developing fetus receives a vaccine given in the mother even though it had neither ingested not been injected with it as described in the Vaccine Act. It was reported that it was concluded in 2001, that the statutory language was ambiguous in its application to this situation, and that both competing interpretations were at least "plausible" by the judge. Therefore, he concluded, that he was not free to choose the interpretation that he found to be more persuasive. He was bound under the cases, rather, to choose the interpretation that would produce the most narrow and restricted waiver of sovereign immunity. In light on the opinion, however, the judge stated that he was free to weigh relative merits of the two competing interpretations. Further, decision specified that he was free to consider general principles of statutory construction other than the sovereign immunity canon of narrow construction, he could take into account the statutory consideration of liberal construction of remedial statutes. The special master directed the patient's parents to determine whether they could obtain an expert report that the vaccine likely progressed through the patient's mother body into the fetus she was carrying and, if so, whether it caused the patient's seizure disorder, brain malformation and significant developmental delay. The representing parent patient's information was reported. Follow up information has been received from an an article via company representative referring to a female infant of unknown age on 20-MAR-2019. Information about medical history, concurrent condition and concomitant therapy was unknown. On 07-DEC-1995, the mother delivered her first via cesarean section. The patient's older sibling was born full-term, and no congenital abnormalities were noted. The mother became pregnant with last menstrual period (LMP) of 15-FEB-1996 and estimated date of delivery (EDD) of 21-NOV-1996. On 25-MAR-1996 in the first trimester of her pregnancy, the mother was vaccinated with M-M-R II (HSA) (dose, frequency, route, batch/lot number and expiration date were not provided) for prophylaxis. The patient experienced exposure to M-M-R II (transplacentally)(foetal exposure during pregnancy, first trimester)(It was also reported that the patient received the vaccination approximately 2 months into her second pregnancy.). On an unknown date during the pregnancy, confirmatory measurements performed by ultrasound showed the actual delivery date of a full term infant would place this immunization at approximately 26 days after conception. On 19-AUG-1996 approximately 27 weeks into her pregnancy: the mother's first prenatal visit took place. On 28-AUG-1996 and 06-NOV-1996, ultrasounds were performed, and the results were unremarkable. The mother delivered the female patient by repeat cesarean due to cephalopelvic disproportion and ineffectual induced labor. The patient's weight was seven pounds, nine ounces. The patient's scores were 8 and 9. On an unknown date upon discharge from the hospital, the patient was noted to be large for her gestational age and macrocephalic. When the patient was two days old, a neonatal sonogram was performed and showed that the patient had enlarged brain ventricles and possible associated cerebral or mid-brain abnormalities. On that same day, the patient was transferred to another hospital for evaluation of possible hydrocephalus. On an unknown date while in the second hospital, the patient received a hearing screening, a computerized tomography(CT) scan, and a cranial ultrasound. The patient was discharged with a diagnosis of hydrocephalus ex vacuo with insult occurring possibly in utero, and was noted to have had an absent corpus callosum. An eye examination revealed hypoplasia of the optic nerves with retinal hypertrophy, dysgenesis and macular sparing. Serology reports showed a normal complete blood count. The patient had positive IgG titers to rubella at 2.49(k) and herpes type 1 at 3.25(f), and negative titers to toxoplasmosis and Cytomegalovirus (CMV). On 27-NOV-1996, the patient was discharged and was scheduled to follow up with neurosurgery and ophthalmology. On 09-DEC-1996 and 29-JAN-1997, the patient was evaluated by her pediatrician and no abnormalities were noted. On 29-JAN-1997, the patient had a slightly bulging fontanel. In approximately mild March 1997, the patient began having episodes of jerking and colonic movements three times a day, followed by being tired and sleepy. On 10-APR-1997, some spells occurred when the patient turned off to the side for about a second at a time for about 15 times in row, and it was concerned that the patient was having seizures by the pediatrician. The patient had an appointment with a physician in the next week and would probably get an electroencephalogram (EEG). In early May of 1997, the patient began to have episodes of severe and prolonged jerks with eyes shifting back and forth lasting for 5-10 minutes. On 12-MAY-1997, the patient had become more severe and prolonged three to four days prior. The patient was admitted for hydrocephalus and to rule out tumor. On 12-MAY-1997, the patient underwent a magnetic resonance imaging (MRI) without contrast, a CT, and an EEG of her brain, which showed agenesis or partial agenesis of the corpus callosum, at least one interhemispheric cyst, and compensatory dilation of both occipital horns, among other things. There was no evidence of a brain tumor. A retinal exam after dilation showed whate areas around each disc with sharp borders, consistent with the lesion seen in Aicardi syndrome(AS). On 15-MAY-1997 upon discharge from hospital, the patient was diagnosed with infantile spasms, brain malformation, bilateral otitis media, developmental delay, diaper rash, and AS. The patient was started on phenobarbital and adrenocorticotropic hormone injection treatment, which ultimately decreased the frequency and severity of the seizures. On 01-JUL-1997, the patient had been no seizures since discharge on 05-DEC-1997. It was noted that although the anterior segment of each eye was normal, but the funduscopic examination did show characteristic fundus lesions of Aicardi's syndrome. On 07-OCT-1997, it was noted that the patient had AS and that she continued to have about 10 sets of spasm-like episodes per day. On 03-DEC-1997, the patient had responded somewhat to Adrenocorticotropic hormone (ACTH), although she still had as many as ten mild clusters a day until recently. On 06-OCT-1998, when the patient was almost two years old, the infant had a history of AICARDI syndrome and infantile spasms. The infant was cruising but was not walking independently. The infant's verbal development was a little more advanced than her gross motor skills, with perhaps 10 words, a good listening vocabulary, good social interaction, and a sense of humor". On 11-DEC-1998, the patient was re-admitted to hospital for placement of a ventriculoperitoneal shunt, in order to relieve intraventricular pressure. The patient was noted to be more alert and more verbal. On 12-JAN-1999 and 24-AUG-1999, the patient was again noted to have been doing well. On 10-JAN-2001, the patient was seen for a fever. The patient was recently seen at the Emergency Room(ER) for a breakthrough seizure. The patient was acute viral syndrome and AS with seizure disorder. On 15-AUG-2002, the patient was taken to hospital because she was having seizures. the patient's was prescribed an increase in phenobarbital. On 22-APR-2004, the patient had been fairly stable since her last visit. The patient was had been taking 120 mg of phenobarbital a day and 500 mg of lamotrigine (LAMICTAL) twice a day. The patient's condition further stabilized, and at a follow-up appointment on 29-JUL- 2004, the patient was having only two or three brief seizures a week. The patient was recommended decreasing her phenobarbital to 105mg a day in divided dosages. The patient returned 03-FEB-2005. The mother complained that the patient had periods of behavioral outbursts and questioned whether her shunt was working properly. During the exam, the patient was uncooperative and had poor eye contact. A CT scan was recommended, and the patient's prescribed phenobarbital dosage was decreased due to infrequent seizures (occurring only once every two weeks). The CT scan results from 07-MAR-2005 showed hydrocephalus (more pronounced on the left side) and indicated that the shunt tube may not be working. The patient was seen at a Neurology Center on 06-APR-2006. The patient's EEG results from January 2006 were described as profoundly abnormal and the patient was noted to be symptomatic of secondary generalized epilepsy syndrome. Other medical impressions were AS, West's Syndrome, Mental Retardation, Cerebral Palsy, and possible Lennox-Gastaut Syndrome. The patient's seizures were poorly controlled and the patient was resistant to medication. On 28-AUG-2007, the patient was prescribed an increase in her Lamictal to 200mg twice a day. On 30-APR-2008, the patient was noted to be stable and her medication was tolerated well. The working impression of the patient's condition was Lennox-Gastaut Syndrome with a history of pharmacoresistant epilepsy. The patient returned to the Neurology Center on 30-SEP-2008, the patient was indicated that had a remarkable reduction in the frequency of breakthrough seizures on the medication topamax. On 15-FEB-2010, an EEG resulted in findings consistent with a diffuse underlying disturbance of neuronal function with superimposed interictal features of a secondary generalized epilepsy such as that can be seen in Lennox-Gastaut syndrome. On 21-JUN-2012, the patient's diagnoses included AS and chronic seizure disorder (now only on one medication). The physician also noted that the patient was having an increasing amount of interaction with family members and more difficulty with discipline. At her follow-up visit on 16-AUG-2012, the patient was still having seizures, as well as daily partial spells. The patient was also having grand mal seizures during her menstrual period. An increase in her anticonvulsant medication, banzel, to three a day, appeared to decrease the number of generalized seizures. On 13-MAY-2013, the patient was noted to have mental retardation, AS and frequent seizures. The patient's medications included medroxyprogesterone acetate (DEPO-PROVERA) and rufinamide (BANZEL). The outcome of foetal exposure during pregnancy, first trimester was recovered. The outcome of other events was unknown. The causality assessments between all the events and ineffectual induced labor and M-M-R II were considered as related. Upon internal review, aicardi's syndrome, Lennox-Gastaut syndrome and seizure were determined to be medically significant. Literature report: Judge in favor of petitioner. Mother case was captured into US-009507513-1903USA011163.; Sender's Comments: US-009507513-1903USA011163:
https://search.brave.com/search?q=Aicardi-Gouti%C3%A8res&source=desktop&summary=1&summary_og=a052a4042c9884efd08ef1
Fang L, Ying S, Xu X, Wu D. TREX1 cytosolic DNA degradation correlates with autoimmune disease and cancer immunity. Clin Exp Immunol. 2023 Mar 24;211(3):193-207. doi: 10.1093/cei/uxad017. PMID: 36745566; PMCID: PMC10038326.




Thanks to Dr J who is unafraid to defend the Truth. A brave and mentally astute woman any cannot but admire and cherish and even love. Truth is a noble virtue and those who defend it are few.
So.. More evidence that you have to be NUCKING FUTS to allow anyone to inject these mRNA products including the flu vaxx and soon everything on the child vax schedule into you or your KiDS!!!