99.7% of adverse events reported in the context of expired products are NOT serious
This aligns well with the idea that degraded products are not inducing AEs or SAEs. But...
I decided to put my poor article of redox shifts and COVID on hold one more time to write this latest tidbit up. I think it’s really important.
I am preparing a presentation for a Public Hearing in Brazil on the 10th of August (I will be presenting remotely), and one of my slides includes the absolute counts of reports from VAERS for the people who reported having been injected with expired product, aka “Expired product administered”. I have been looking at this for ages but as my brain would have it, I never looked at the ratio of AEs to SAEs before today. So I looked. I was amazed.
99.7% of the 37,282 reports are considered not to be serious adverse events. To be clear, a serious adverse event is one where death, hospitalization, an ER visit, disability, birth defect or life threatening illness occurred. The report is usually made in temporal proximity of the injection date.
In the presentation I am preparing, I address the issue of modified mRNA injectable product degradation and pose two questions:
Are expired batches of the COVID-19 injectable products being injected into people?
Is this resulting in more or fewer reported SAEs?
The follow-up slide answers both questions very readily. Expired batches of the COVID-19 injectable products are being administered and reported to VAERS at high rates (1,155,742/676,627,865 = 1/585 → using an URF of 3112). This means that according to an URF of 31, 1 in 585 people have been injected with expired product. Interesting.
This is an incredible result. I decided to look more closely at the details of some of these reports. One of the details I wanted to check was the timeframe from injection to onset of the report. The onset of the report would be ‘immediately’ (day 0) in almost all cases, logically, since most of these reports would be promptly filed by the administrator of the shot, having immediately realized that they had made an oopsy. Perhaps immediately after injection, the administrator checked the vial label and noticed that the expiration date for that vial and/or batch had passed. I plotted the percentage of reports filed against the timeframe between injection and onset set.
94% of these reports were filed immediately. So the oopsies were caught right away and reported. One problem I could anticipate from this is that since follow-up reports for people who already have a permanent VAERS ID are not completed, then if any of these people actually did get, say, myocarditis, VAERS would likely never reveal that. All VAERS would reveal are these non adverse event adverse event reports. If you know what I mean. “Expired product administered” is the MedDRA code used and it is considered to be an adverse event, but it’s not actually a side effect: it’s an administration error. It actually occurs to me, that this might even be a tactic to hide yet even more adverse events or serious adverse events, but, this is pure speculation.
So, one might wonder, if these aren’t actually reports of adverse events, then why would there be any associated reports of hospitalizations or deaths or any other serious adverse event? Well, because of what was in the syringe.
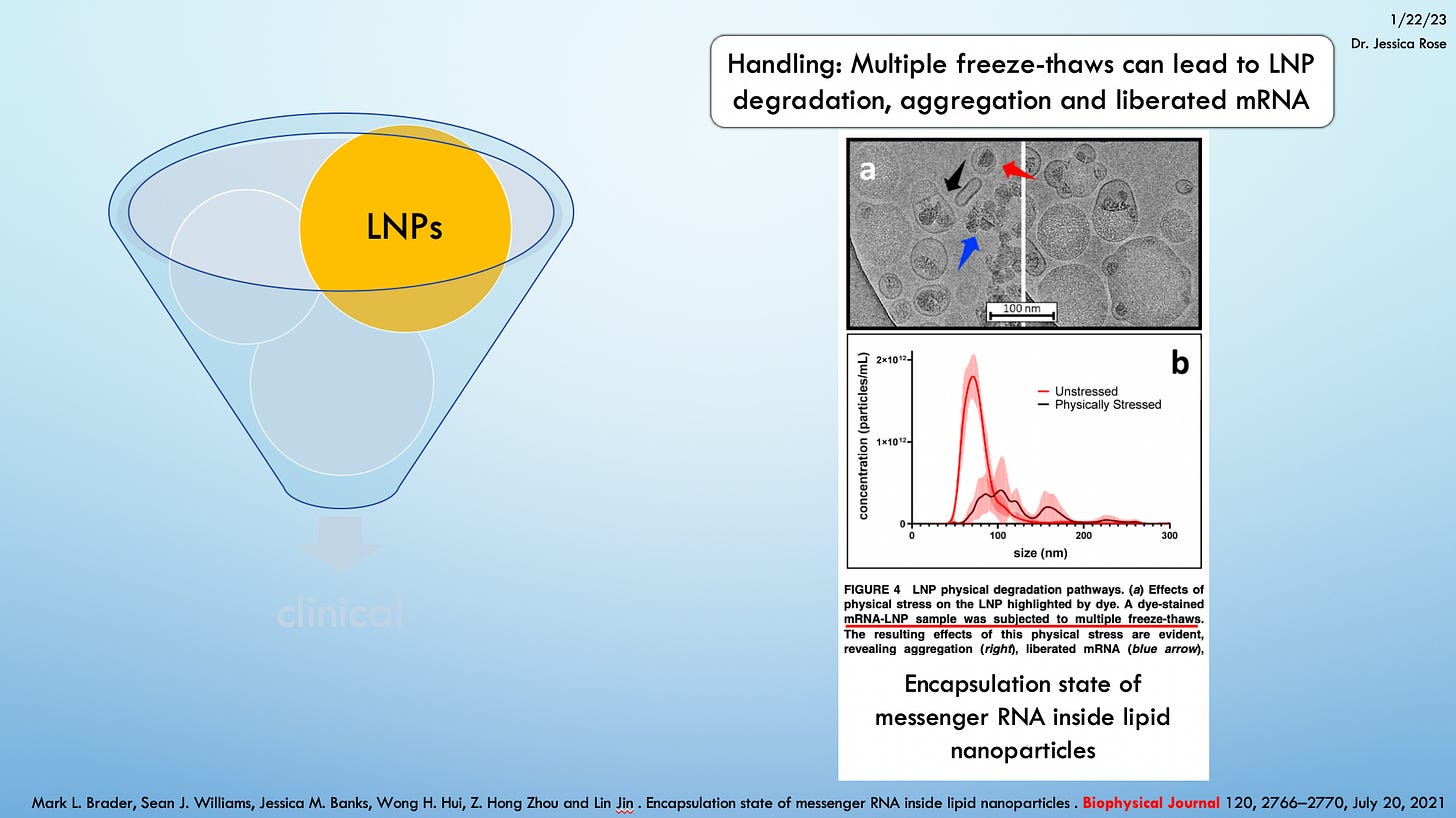
Despite the fact that the product was expired, it was still injected and for all we know, because quite frankly, we know nearly nothing after 3 years, these expired products could actually induce more damage than the non-expired products. Perhaps the degraded LNPs could make for aggregated product that could gum up the works once injected?
The fact remains that there are very few associated reports of death (12), hospitalization (24), ER visits (62), disability (14), birth defect (0) or life threatening illness (7) in the context of these expired shots.
It should be noted that one of the people who died (VAERS ID: 1789626) was only 22 years old - a female from Mexico and she had no listed counter-indications and no history of disease. She died from lethal heart arrhythmia and respiratory failure following dose 1 of the Pfizer injectable product. The Vax Lot number (30150BA) is not the typical Pfizer nomenclature which has 2 letters followed by 4 numbers. All of the Pfizer lots with this number were administered in Mexico, so maybe it’s simply the number associated with the country where the product was administered.
I mention her because she matters and because even though most of the reports filed for expired products were not associated with SAEs, some were, and more importantly, without explanation.
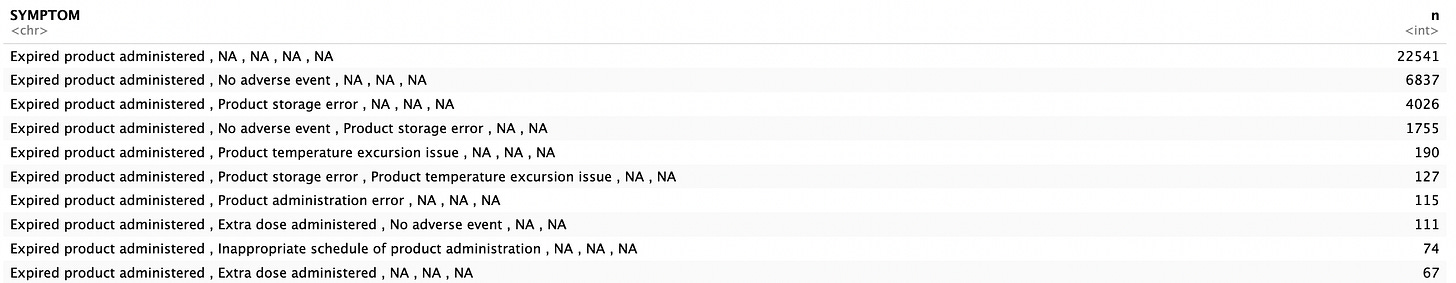
Just to give my readers an idea of the other MedDRA codes listed alongside “Expired product administered”, I made a little table of the top reported sets of AEs. As you can see, most often, the “Expired product administered” MedDRA flew solo. Next in line were reports in tandem with “No adverse event”. Well, maybe not immediately. And the list goes on pertaining to administration buggery. The problem I anticipate here and why I have my suspicions about the reality of the 99.7% of AEs being non-serious is due to the ‘promptness’ of the reporting.
What adverse events did these people succumb to at a later date? Will they have this information included in an updated VAERS report? Probably not. It’s like this: imagine you slip and fall and hit your head and someone picks you up immediately and asks you if you’re ok. You’re probably going to say yes, even though it turns out you have a concussion. You would definitely have something other than ‘yes’ to say as an answer to the question: ‘Are you ok?” a week later. But that someone who originally asked you is nowhere to be seen. You will always be ‘fine’ in their eyes. Meanwhile, you’re dizzy and can’t concentrate or sleep. Your life is disrupted.
I see it like this. So even though 99.7% of the [immediately filed] reports associated with expired products are not associated with SAEs, I dare to ask the question: what would this rate be if we queried a month later?
It might be the same.
It might be completely different.
For now, it appears as though it’s a good thing to be injected with expired product. But, and this is a big but (I like big buts), this could be an illusion.
Rose, J. 2021, Critical Appraisal of VAERS Pharmacovigilance: Is
the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System? Science, Public Health Policy & the Law
Volume 3:100–129.
https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total








Entertaining. If it weren't about human lives. Here in Italy the deadline was extended by law. Expired lot? No! Just one stamp and it lasts another six months and then six more months. Too bad that for more than a year the data of adverse reactions have not been published (and it seems that it is common to many European countries). But, on the other hand, what is the use? Vaccinations are for killing and they are trying them in various combinations. More graphene less graphene, more peg less peg, more rmna less rmna, fresher more expired etc. When dr. Mengele will have the right data another vaccine will arrive
It gets more evil by the hour... Keep on exposing