Update (Dec.13, 2023): These products and the subsequent untested technology got approval this week by Japanese authorities. You can read about that here.
Please head to the document entitled: “INVESTIGATOR’S BROCHURE BNT162/PF-07302048” dated August 12, 2020.
About half a year ago, I suppose, I virtually sat across from a Canadian researcher who was developing a new technology called self-amplifying RNA (saRNA). If you want, you can watch that interview here. My hosts are lovely people. At the time, I found it difficult to listen to my co-interviewee because a) I used to be her and b) I know the dangers associated with what she is developing. It is my opinion that she did not.
I revisited this interview whilst writing this and it is good. How frikkin’ diplomatic am I?
“Why do the best solutions and advances, get thrown away into the worst possible hands?” Jello Biafra. I was sent a video made by Jello showing that he has completely sold out. How very sad how my heroes fall one-by-one. What happened to punk?
So the claim is that this self-amplifying messenger RNA is awesome because you need to inject less of it. But here’s the thing: it encodes its own replicase enzyme - it makes copies of the RNA (whatever it is) once inside the cells. Does that sound problematic to you? To me, it does. And here’s why. If the other genes in the mRNA encode destructive proteins (like the modified mRNA of the SARS-nCoV-2 spike protein), then… for one, how will it ever be possible to turn off these self-amplifying RNAs? Are the replicases self-limiting?
If you ask me, the people developing and promoting this technology are concerned only about profit, ‘cost’ and initial dose. They claim that adverse event occurrences are proportional to the initial dose of RNA. Hmm. Anna claims in the interview that the menstrual cycle disruptions induced by the COVID-19 mRNA injectable products are temporary, and can also be brought on by viral infections. She also claimed that the vaccinated people won’t get severe COVID-19 in the context of hospitalization. Really? I missed out on that point in the interview. She was pretty sure that since most of the people in Israel had been injected, that this would explains why the majority of COVID-19 hospitalizations were in the context of the injected. But then, if the shots were meant to reduce severity, why would they be ending up in the hospital?
On saRNA
So as we all know by now, the messenger RNA in the COVID-19 Pfizer/BioNTech and Moderna products, is the mode of delivery of information for the cells in our bodies to make lots and lots of modified spike proteins. That’s what these folks are now calling ‘conventional’ mRNA.1 Well now! That was fast. Now it’s a CONVENTION? Aaaaaaaaaaalrightttttttyyyyy thennnn.
So do you see the highlighted bit in green? That’s the code for nsP1-4 genes that, following in situ translation, form an RNA-dependent RNA polymerase (RdRP) complex resulting in accumulation of antigen within the cell. The nonstructural proteins 1, 2, 3, and 4 (nsP1-4) are essential for replicon activity as they form the RdRP complex.2 The ‘conventional’ modified mRNA is non-replicative because it does not include an RdRP (RdRPs these take pieces of RNA and make copies of it). These enzymes are very specific to the RNA. The UTRs help to maintain this specificity. Apparently, no human RdRP can do this for the conventional RNAs. The saRNAs do replicate, however. And apparently, again, the RdRP that comes with it, is the only one that will allow this to perpetuate. Chicken and an egg?
Here are a few sources of information about this technology.3 4 5 6 7 8 9
Onto the document. Please skip ahead to page 13.
So this saRNA has been on the schedule the whole time. They didn’t even anticipate the possibility that the ‘conventional’ mRNA would be a failure, because, if it was, imagine how bad that would be for the schedule! And guess what, it’s being trialed now.
What do you want to bet that those trial participants have NO IDEA that they are being injected with self-amplifying RNA? This study is active with ‘estimated’ primary completion date April, 2023 (final data collection date for primary outcome measure).
BNT162c2 will be administered using a Prime/Boost (P/B) regimen. The vaccine BNT162c2 will also be administered using a Single dose (SD) regimen.
Experimental: BNT162c2 (P/B) - Part A 18-55 years of age
Escalating dose levels
Intervention: Biological: BNT162c2.
The primary endpoint is 21 days post injection.
Actual Enrollment (submitted: January 12, 2022): 512
I find two things to be insane:
That I haven’t heard that the saRNAs are already being trialed and
That there is no mention of self-amplifying mRNAs in the clinical trial data sheet. There is, however, a very long exclusion criteria list and an informed consent clause as part of it.
Inclusion criteria: Have given informed consent by signing the informed consent form (ICF) before initiation of any trial-specific procedures.
What do you want to bet that this document that required signing said nothing of self-amplifying RNAs?
I put this together today when I opened up the FOIA document attached at the top of this article. I had received an email on June 9th, 2022 (10 days ago) that was addressed to myself and Robert Malone as to what the ‘BNT162c2’ was. I had told the addresser that I hadn’t heard of it before and Robert pointed us both to the clinical trial with ClinicalTrials.gov Identifier: NCT04380701.
But, I still didn’t know what it was. Maybe it had a different LNP? Nope.
It’s a completely different type of technology. Again.
What’s the encoded antigen? We’re not going to tell you! But we are going to insist that you get injected with it. Or, no soup for you.
Even though there are peer-reviewed studies on this technology, I cannot stress this point enough: WE NEED LONG TERM DATA WHEN IT COMES TO NEW TECH. Not 21 days. Not 365 days. We need years. We are NOT in an emergency and therefore, we have the time to explore the safety and efficacy of saRNA technology carefully and properly. Like we should have been doing the whole time with our now magically ‘conventional’ mRNA tech.
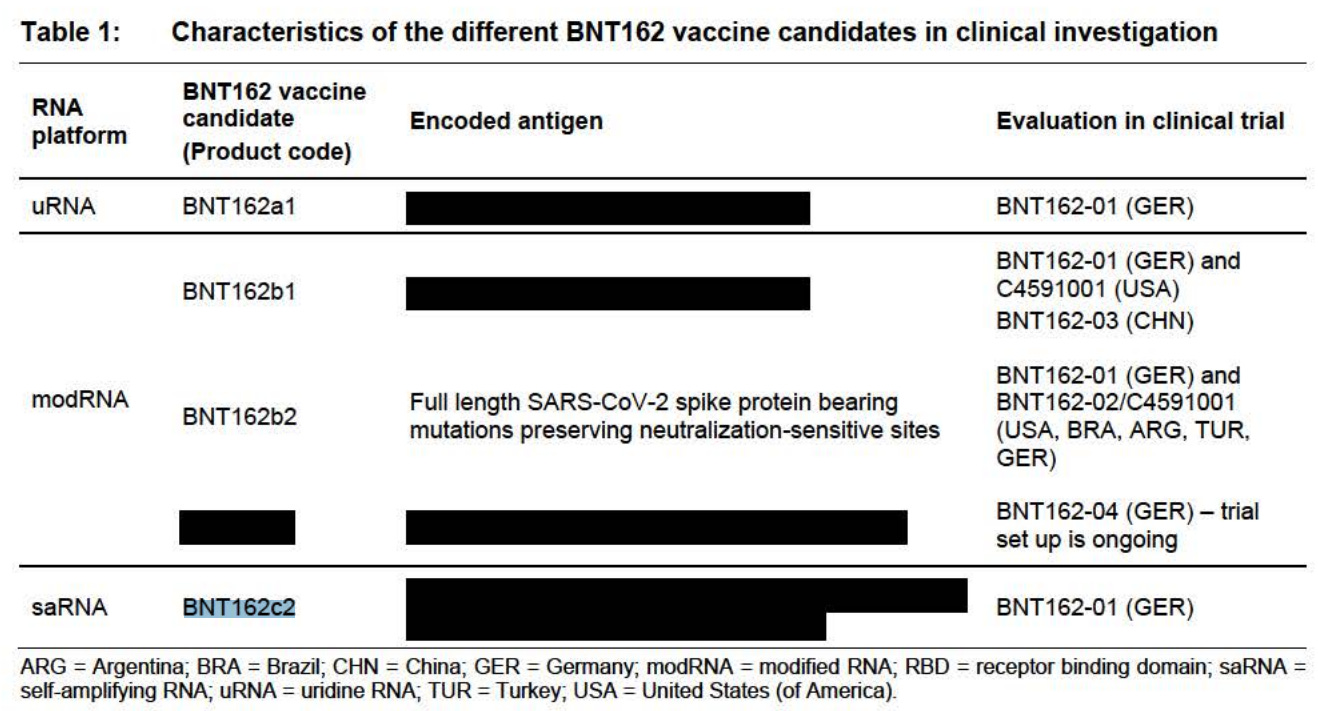
Sorry, I have to include this screenshot. This is just too precious. It’s so safe. Just inject me now. Wait, I can’t see! Everything turned black! Kind of like the safety data redaction for the product. (Sorry for the very dark humor. Sorry. I did it again.)
Oh and they mention in section 7.2 Posology and method of administration, that ‘the vaccine should not be injected into areas where there may be a major nerve trunk’. Ok. Good to know.
And this literally made me LAUGH OUT LOUD. Look at this.
7.11.2 Non-clinical findings of note
All tested non-clinical and clinical vaccine candidates were immunogenic to highly immunogenic in non-clinical models. The available data demonstrate that BNT162b1, BNT162b2, BNT162b3, and BNT162c2 are capable of inducing robust immune responses in mice, (except for BNT162c2) rats and NHPs.
Why would they include BNT162c2 in this list if it’s not in the list? Or did they mean for the rats, and not the mice?
So when you order a cheeseburger, because you love cheese, and it comes without cheese on it and you complain, they yell at you that ‘cheeseburger’ is just a word and had nothing to do with a burger having cheese on it, what are you to do? Same logic, yeah?
If we have not learned yet that we need to be cautious with gene-based therapies, then, you know what? I just don’t know what to say.
Bloom, K., van den Berg, F. & Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther 28, 117–129 (2021). https://doi.org/10.1038/s41434-020-00204-y.
Kääriäinen L, Ahola T. Functions of alphavirus nonstructural proteins in RNA replication. Prog Nucleic Acid Res Mol Biol. 2002;71:187–222.
Maruggi, G., Ulmer, J. B., Rappuoli, R., & Yu, D. (2021). Self-amplifying mRNA-Based Vaccine Technology and Its Mode of Action. Current topics in microbiology and immunology, 10.1007/82_2021_233. Advance online publication. https://doi.org/10.1007/82_2021_233.
Brito, L. A., Kommareddy, S., Maione, D., Uematsu, Y., Giovani, C., Berlanda Scorza, F., Otten, G. R., Yu, D., Mandl, C. W., Mason, P. W., Dormitzer, P. R., Ulmer, J. B., & Geall, A. J. (2015). Self-amplifying mRNA vaccines. Advances in genetics, 89, 179–233. https://doi.org/10.1016.
Geall, A. J., Verma, A., Otten, G. R., Shaw, C. A., Hekele, A., Banerjee, K., Cu, Y., Beard, C. W., Brito, L. A., Krucker, T., O'Hagan, D. T., Singh, M., Mason, P. W., Valiante, N. M., Dormitzer, P. R., Barnett, S. W., Rappuoli, R., Ulmer, J. B., & Mandl, C. W. (2012). Nonviral delivery of self-amplifying RNA vaccines.
McKay, P. F., Hu, K., Blakney, A. K., Samnuan, K., Brown, J. C., Penn, R., Zhou, J., Bouton, C. R., Rogers, P., Polra, K., Lin, P., Barbosa, C., Tam, Y. K., Barclay, W. S., & Shattock, R. J. (2020). Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nature communications, 11(1), 3523. https://doi.org/10.1038/s41467-020-17409-9. (Notice the conflicts here.)
Ballesteros-Briones, M. C., Silva-Pilipich, N., Herrador-Cañete, G., Vanrell, L., & Smerdou, C. (2020). A new generation of vaccines based on alphavirus self-amplifying RNA. Current opinion in virology, 44, 145–153. https://doi.org/10.1016/j.coviro.2020.08.003.
Blakney, A. K., McKay, P. F., Hu, K., Samnuan, K., Jain, N., Brown, A., Thomas, A., Rogers, P., Polra, K., Sallah, H., Yeow, J., Zhu, Y., Stevens, M. M., Geall, A., & Shattock, R. J. (2021). Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines. Journal of controlled release : official journal of the Controlled Release Society, 338, 201–210. https://doi.org/10.1016/j.jconrel.2021.08.029. (Notice the conflicts here.)
Lundstrom K. (2020). Self-Amplifying RNA Viruses as RNA Vaccines. International journal of molecular sciences, 21(14), 5130. https://doi.org/10.3390/ijms21145130.











Stop doing this to me. Only have so many ‘Holy Crap’ in me Miss Rose! 👀🤷♂️💉
I’m not a scientist and I don’t play one on TV, so forgive any ignorance or misunderstanding here. However, I did just listen to a CHD TV video interview, which included Michael Palmer, MD. He suggested (start at 1hr 26 min time stamp) that the saRNA may exist in these shots already — an EARLY CLINICAL TRIAL perhaps? See: https://live.childrenshealthdefense.org/shows/chd-friday-roundtable/Aq7JQ72WgN
The entire Roundtable is worth watching — horrible stuff of course — but this late question from Brian Hooker, PhD, sparked the scary potential explanation for the persistence of spike protein in the vaxxed.