Morty kinda had big pills for breakfast! And you! Who saved room for pill-brûlée!
My Master’s degree was an incredible part of my academic career. I studied HIV immunopathogenesis using dynamical systems analysis. I was lucky enough to work in the Biosafety Level III lab where I did experiments using the blood and plasma from people who were carrying HIV and at different stages in their ‘disease progression’. You can read my thesis here.
The title of my thesis was:
Dynamical Systems Analysis of HIV Immunopathogenesis and the Effects of Antiretroviral Treatment Interruption.
I believed through and through that these people would be able to take regular scheduled breaks from their multi-drug antiretroviral treatment regimens - not only safely - but with the benefit of triggering boosts of HIV-specific CD8+ T cell activity to keep the virions at bay. I did not entirely succeed in my quest but I learned an enormous amount.

Here is the abstract.
In the absence of a successful vaccine against HIV-1, alternative means to cope with the HIV pandemic have been explored. Antiretroviral (ARV) treatment is commonly prescribed to HIV-infected individuals in an attempt to control the infection. However, ARV treatment is very rigorous and generally comprises a highly toxic and costly multi-drug regimen designed for strict, life-long adherence. ARV treatment interruption is an alternative treatment strategy designed to maximize clinical benefits and alleviate some of the complications associated with continuous treatment. It is theorized that treatment interruption can reset the virological setpoint by inducing controlled resurgences of virus to boost HIV-specific CD8+ T cell activity to control viral replication.
Dynamical systems analysis is a mathematical tool used to describe the behavior of complex systems. This allows an unobtrusive, safe way to test treatment interruption regimens. I have developed a novel mathematical model that describes the dynamical interactions between HIV-specific T cell and virus populations for two clinically-defined subgroups of HIV-1-infected individuals called fast and slow progressors. The model is based on antiviral activity imposed by HIV-specific CD8+ T cells that specifically target the virus by removing virally-infected cells and the model accurately mimics clinical disease progression patterns in these subgroups. Model results accurately predict that treatment interruption induces resurgence of virus to boost HIV specific CD8+ T cell activity in both subgroups, but does not reset the virological setpoint in either. These results are experimentally corroborated through an assessment of quantitative and qualitative changes in HIV-specific CD8+ T cell activities when virus loads are high (off-treatment) and low/undetectable (on-treatment).
In this thesis, I provide an introduction to HIV and dynamical systems, a
complete analysis of the model derived to describe HIV immunopathogenesis,
a report on clinical and experimental observation of a small cohort of HIV-1-
infected individuals and a description of the integration of the mathematical,
clinical and experimental results.
Let’s go back to that part about ARV.
Highly toxic
Costly
Multi-drug regimen
Strict, life-long adherence (with HIV)
There were four major classes of ARV drugs on the go at the time I wrote my thesis: nucleoside analogue reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs) and fusion inhibitors (FIs). These drugs act at different points during the infection/replication process but all suppress viral replication. FIs prevent the host cell from becoming infected. NRTIs and NNRTIs prevent the provirus from being produced. PIs on the other hand act subsequent to infection of the host cell to prevent the formation of new virions. The toxicity of Highly Active Antiretroviral Treatment (HAART) can lead to secondary health problems and the necessity for additional prescription drugs to control clinical complications. In addition, antiretroviral drugs are quite costly to manufacture and distribute.
At that time, the multi-drug regimen included a combination of a PI, an NRTI and an NNRTI. Fusion Inhibitors were the new thing back then. PIs block viral proteases: they act by binding to the catalytic site of the (HIV) protease, thereby preventing the cleavage of viral polyprotein precursors into mature, functional proteins that are necessary for viral replication.1
Now a little about our new P-fizer miracle drug P-f-axlovid.
Paxlovid is a combination of nirmatrelvir and ritonavir2 and has been granted our now infamous Emergency Use Authorization (EUA) for off-label use in the context of COVID-19. It seems nowadays that no pharmaceutical or biological product need surpass the EUA anymore, according to FDA ‘standards’.
Nirmatrelvir: Nirmatrelvir is an orally bio-available PI.3 On December 22, 2021, the FDA issued an EUA for ritonavir boosted nirmatrelvir for the treatment of patients with mild to moderate COVID-19 aged ≥12 years and weighing ≥40 kg who are within 5 days of symptom onset and at high risk of progressing to severe disease.4 5 6 7
Ritonavir: Ritonavir is an ARV PI that is widely used in combination with other PIs in the therapy and prevention of HIV.8 This drug is highly hepatotoxic (injures liver) and the mechanism of injury is unknown.9 10 11 12 Other side effects include nausea, diarrhea, gastrointestinal upset, change in taste, fatigue, rash and, with long term use, hyperlipidemia and lipodystrophy. These are the kinds of shitty side effects I was hoping to help people on HIV meds avoid by taking these drugs. You can see other lovely side effects listed here.
The pharmacodynamics of lopinavir/ritonavir raise concerns about whether it is possible to achieve drug concentrations that can inhibit the SARS-CoV-2 proteases. In addition, lopinavir/ritonavir did not show efficacy in two large randomized controlled trials in hospitalized patients with COVID-19. There is currently a lack of data on the use of lopinavir/ritonavir in nonhospitalized patients with COVID-19.13
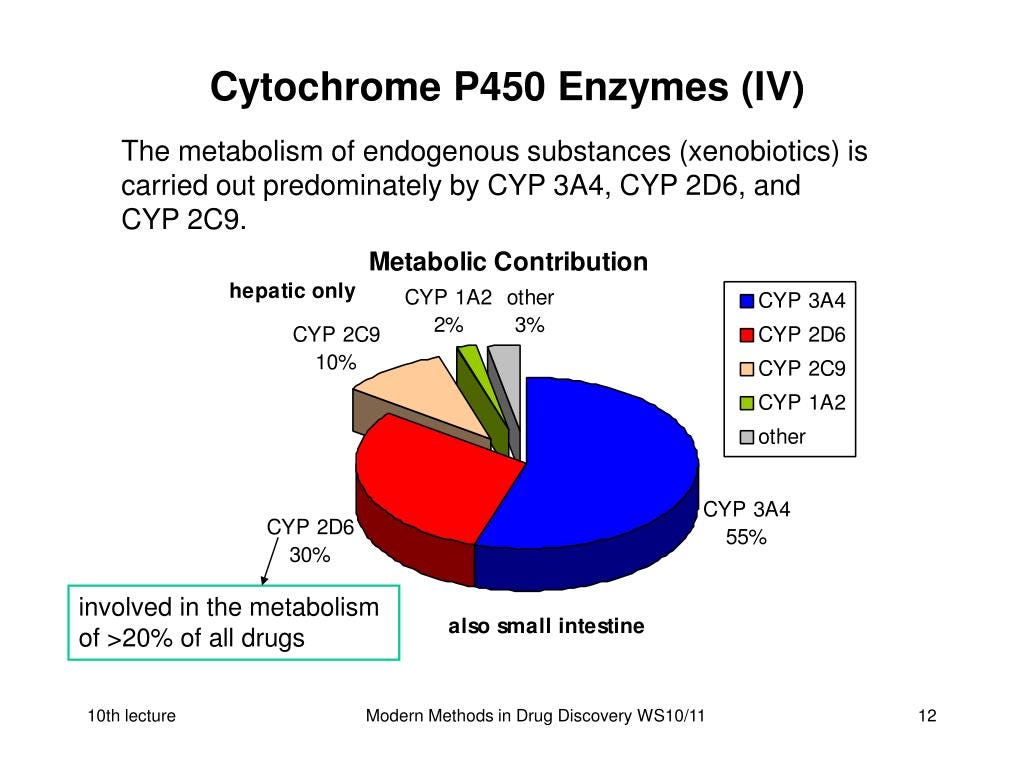
Ritonavir maintains potent inhibition of the cytochrome P450 3A4 isoenzyme which metabolizes endogenous substances.
Let’s be clear here. Ritonivir is not the kind of thing that I think should be used off-label. When I say that I mean re-purposed from ‘use’ in the HIV context versus the COVID-19 context. (Hmm. Think about that.) And considering these mindless bureaucrats keep going psycho about drugs like Ivermectin and Hydroxychloroquine that have decades-long proven safety profiles, in off-label context, you’d think they’d think twice about re-purposing a drug that is KNOWN and PROVEN to be HEPATOTOXIC!14
I think this is literally another ridiculous ploy to profit. P-fizer knows that their injections are pf-toast (at least the COVID-19 ones) and so they need a new and fresh way to keep profits p-flowing.
Take an old, toxic, and expensive pharmacokinetic enhancing, endogenous substance metabolism inhibitor PI15 against HIV, add another PI and presto-changeo! A new anti-COVID-19 miracle pill! Tada! Get yours today! For a coronavirus with an Infection Fatality Rate akin to the flu. Yes. Sounds like a great plan. For the profiteers. If people would do this with condoms, they’d do it with pills. Imagine being the person who had to ‘clean’ them. Shudder.
My bestie Jilly asked me about this drug today so I looked into it. After finding out this is basically ARV, I thought it would be helpful to let people know that taking a multi-drug involved ARV treatment for a coronavirus that is essentially innocuous to healthy people and children is probably not what I would recommend. Maybe I am wrong. You decide. And do read the references. :)
Protease Inhibitors (HIV). (2017). In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases.
https://www.yalemedicine.org/news/12-things-to-know-paxlovid-covid-19
https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/
Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung SH. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem. 2016;59(14):6595-6628. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26878082.
Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586-1593. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34726479.
Centers for Disease Control and Prevention. COVID-19: people with certain medical conditions. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed December 27, 2021.
Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. 2021. Available at: https://www.fda.gov/media/155050/download.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Ritonavir. [Updated 2017 Sep 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548301/
Marzolini C, Stader F, Stoeckle M, et al. Effect of systemic inflammatory response to SARS-CoV-2 on lopinavir and hydroxychloroquine plasma concentrations. Antimicrob Agents Chemother. 2020;64(9). Available at: https://www.ncbi.nlm.nih.gov/pubmed/32641296.
Schoergenhofer C, Jilma B, Stimpfl T, Karolyi M, Zoufaly A. Pharmacokinetics of lopinavir and ritonavir in patients hospitalized with coronavirus disease 2019 (COVID-19). Ann Intern Med. 2020. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32422065.
Group RC. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33031764.
WHO Solidarity Trial Consortium, Pan H, Peto R, et al. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity Trial results. N Engl J Med. 2020. Available at: https://www.ncbi.nlm.nih.gov/pubmed/33264556.
https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/lopinavir-ritonavir-and-other-hiv-protease-inhibitors/
Haas, D. W., Koletar, S. L., Laughlin, L., Kendall, M. A., Suckow, C., Gerber, J. G., Zolopa, A. R., Bertz, R., Child, M. J., Hosey, L., Alston-Smith, B., Acosta, E. P., & A5213 StudyTeam (2009). Hepatotoxicity and gastrointestinal intolerance when healthy volunteers taking rifampin add twice-daily atazanavir and ritonavir. Journal of acquired immune deficiency syndromes (1999), 50(3), 290–293. https://doi.org/10.1097/QAI.0b013e318189a7df
Ritonavir has potent inhibition of the cytochrome P450 3A4 isoenzyme which metabolizes endogenous substances.





Think I'll stick with pine needle tea, zinc, quercetin, melatonin, oregano oil, iodine, selenium, and vitamins C, D, and B-complex. Haven't had a cold since 2010 and am untested, unmasked, unjabbed, and unafraid.
This is a great article! Very many important details. I did NOT know that it was so hepatotoxic.
Another interesting aspect of Paxlovid is that it was tested on UNVACCINATED people. It was not trialed on vaccinated people.
Without spamming links, I may mention that myself and Brian Mowrey wrote Substack articles that discussed one interesting aspect of Paxlovid: it seems to be more of a five-days biomolecular PAUSE button, simply pausing replication for five days.
At around day 10, numerous people (mostly vaccinated) report resurgence of the virus and reappearance of symptoms.
Brian Mowrey looked at this and also came up with a biological mechanism of how this resurgence would happen. Take a look at our posts and see discussion in comments also, especially in comments to Brian's articles.
I believe that Paxlovid is much less efficaceous than Pfizer wants us to believe, which should not surprise us too much given Pfizer's history.
Maybe the three of us can bring attention to the problems of Paxlovid, some of which you outlined but also the post-treatment resurgence, and save some lives by highlighting that Paxlovid is not as good as advertised.
Pfizer deserves the highest degree of scrutiny.