'Safety'? How about we start referring to these as 'Hazards'.
A little birdie sent me an email... keep 'em coming. And way to go team.
Today I received an email from a citizen warrior with two documents attached. By the way, I will post everything you guys send, no matter how ‘sensitive’. We paid for this program and these products, and we not only deserve to know what’s being injected into our species, but are entitled to know. I can’t believe that what I just wrote is deemed ‘controversial’.
The two documents are Pfizer ‘safety’ documents readily available online entitled Pfizer Safety Data Sheet and A phase 1/2/3, placebo-controlled, randomized, observer-blind, dose-finding study to evaluate the safety, tolerability, immunogenicity, and efficacy of SARS-nCoV-2 RNA vaccine candidates against COVID-19 in healthy individuals.
The emailer referred me to page 131 in the latter document where something peculiar is documented.
All SAEs (severe adverse events) were to be reported by fax or phone. There is only one reason to exclude initial notification by formal written documentation for an SAE: to ensure that if a report needs to be ‘erased’, it can be. My emailer put it this way:
Why do they request all SAEs to be transmitted via ONLY facsimile, telephone, and hard-copy mail? Because, in my humble opinion, there then is no electronic record/trail.
I think my emailer is 100% correct. If I was in charge of SAE reports for a questionable ‘clinical trial’ and I wanted to vet the reports such that if, say, there were a lot of deaths being reported in temporal proximity to injecting my tremendously safe products into people, then I would make damned sure that those reports came privately and securely to me (without a paper trail) so that I would never have to fess up to their existence if I didn’t want to, and thus I could always say that my creepy injectable products were safe and effective.
On page 128, they also state the following:
It should be noted that the Vaccine SAE Report Form for reporting of SAE information is not the same as the AE page of the CRF.
They do write that in addition to that phone call, you have to do your paperwork, but, to be litigious, their bestie CDC also claim that VAERS reports are compulsory to file is an AE is even suspected, while simultaneously creating threatening environments in medical establishments for doctors who do want to file to encourage them not to.
And by the way, they are meant to be doing causality assessments as well.
For each AE/SAE, the investigator must document in the medical notes that he/she has reviewed the AE/SAE and has provided an assessment of causality. There may be situations in which an SAE has occurred and the investigator has minimal information to include in the initial report to the sponsor. However, it is very important that the investigator always make an assessment of causality for every event before the initial transmission of the SAE data to the sponsor.
Here’s the second piece of information from the former document. If you refer to the Safety Data Sheet on page 2, you’ll find something called Potassium chloride in the Hazardous ingredients table. In column 5, you’ll note a classification of Acute Tox 5 (H303).

Potassium chloride is a mineral found in many foods and is essential for proper functioning of the beating of the heart. It can be taken as a therapeutic to prevent, or aid in, elevation of low serum levels of potassium in the context of a condition called hypokalemia. Side effects of excessive potassium include uneven heartbeat, muscle weakness or limp feeling, severe stomach pain, and numbness or tingling in your hands, feet, or mouth1 and thus ingestion of this medication should be monitored carefully. In concentrated injected form2, its intended use is for maintenance of serum potassium.
Notice the list of adverse reactions associated with administration of highly concentrated IV potassium chloride in the following Table found on page 6 of the FDA document found here.
You’re not meant to administer the IV concentrated form of potassium chloride if one has a cardiac disorder.
Question: What does this mean for the children who are diagnosed with myocarditis in temporal association with these shots?
They have a diagnosed cardiac disorder. Injection of potassium chloride is contraindicated with diagnosed cardiac disorders. Therefore, knowingly injecting someone with a contraindicated substance is medical malfeasance and that administrator is personally liable.
The dose 2 effect observed in myocarditis in children may be precisely this: the primary cardiac damage exacerbated by the secondary shot due to the presence of potassium chloride. To me, it would be essential that the concentration of potassium chloride was known. As it stands, according to the Pfizer Safety Data Sheet, the ‘Specific concentration limit (SCL)’ is ‘Not Listed’.
They also have Potassium phosphate listed in the NonHazardous ingredients and injection of potassium phosphate is contraindicated with hyperkalemia.
I looked H303 up in the GHS Classification on Pubchem.
The reason why data is missing in a classification of toxicity is usually because the data has not been collected to date and therefore nothing is actually known about the toxicity at the time of publication of the document. “No data available.” Funny that. They put this stuff into 5 billion people before the toxicity data was available and said screw the environment. But, climate change.
The classification of this chemical starts with an ‘H’ and all of the Hs look pretty bad according to the Pictograms. It says that it’s bad if you eat it but, there’s no Pictogram associated with it yet as shown in Figure 4. This to me means, they don’t know how to classify it yet. There is also a warning attached to it.
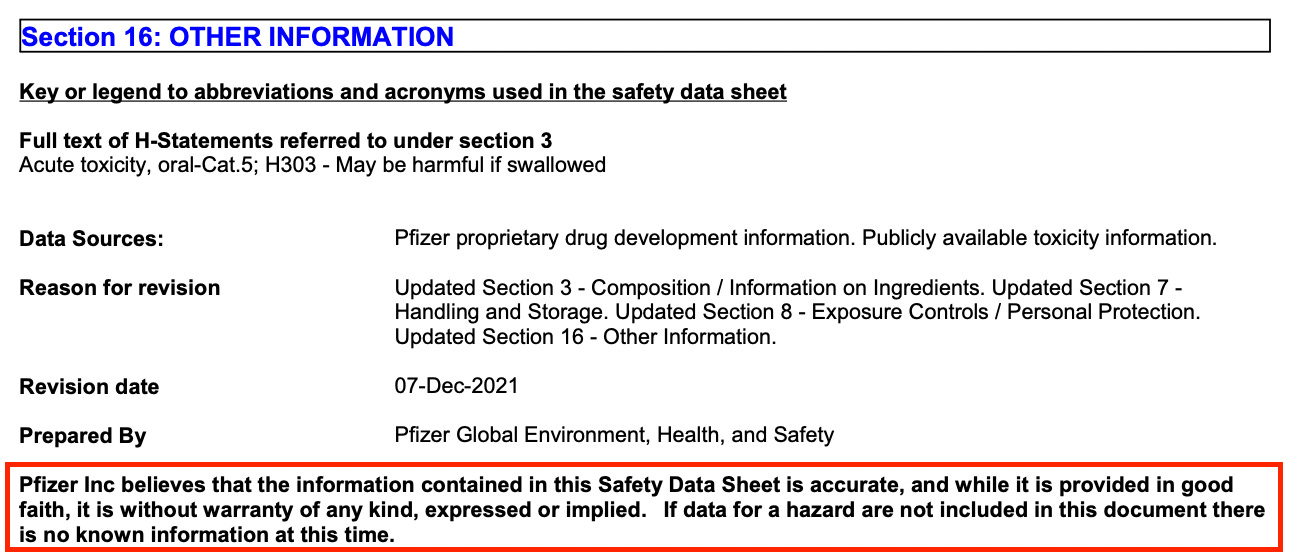
Pfizer ‘in good faith’? Are you kidding me? Pfizer has paid out the most in health care fraud settlements in history3 “to resolve criminal and civil liability arising from the illegal promotion of certain pharmaceutical products” according to the United States Justice Department. THEY’VE ALREADY BEEN CAUGHT RED-HANDED HARMING THE AMERICAN PUBLIC! What more do people want? When they claim that the information they provide to the public is ‘in good faith’, I say, alrighttyyyyyy thennnnnnnnn. And when they subsequently claim that un-included hazard data is ‘not known at this time’. I say, alroooioightyyyyyyy thennnnnnnn. Not known to us suckers anyway.

I may not be up-to-date on what a poison is, but I would rather not get injected with something with the word poison associated with it.
How about you?
Thank you to my emailer.
https://www.drugs.com/potassium_chloride.html
https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019904s014lbl.pdf
https://www.justice.gov/opa/pr/justice-department-announces-largest-health-care-fraud-settlement-its-history







"Pfizer has paid out the most in health care fraud settlements in history3 “to resolve criminal and civil liability arising from the illegal promotion of certain pharmaceutical products” according to the United States Justice Department. "
Honestly, NO fine can do justice; they need to be disbanded. They were awarded the same status as the most crooked banks: "too big to fail"... Says who?
So basically, if one ingredient in these death shots doesn't kill or maim you, another one -- or all of them mixed together -- will.
Jessica Unacceptica -- Huge thanks to you and your readers for digging into all the messy stuff the rest of us never will!
------
A teensy favor please (בבקשה -- pronounced Bevakašá -- in Hebrew, I looked it up):
בבקשה define all acronyms before using them for us lay folks, even if you think we should know them by now; new people join your crucial crusade every day:
* Adverse Event (AE)
* Severe Adverse Event (SAE)
* CRF?
"It should be noted that the Vaccine SAE Report Form for reporting of SAE information is not the same as the AE page of the CRF." What is CRF in this context? https://www.allacronyms.com/CRF