RSV reports in VAERS (AREXVY and ABRYSVO)
Is there a safety signal in pregnant women already?
I’ve written about the RSV vaccines already and you can read about that here.
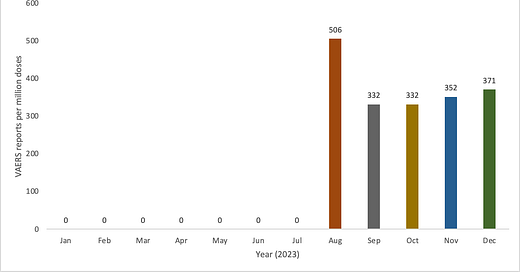
Now that these products have been deployed, we are already seeing reports of AEs and SAEs being reported to VAERS. 68% of the reports have been filed in the context of the Arexvy product. To date, there are 2,903 VAERS IDs reported with a 13% SAE reporting rate whereby 38% of the reports were filed on the same day as the vaccine was administered. The average age of the reporter is 70 which makes sense because these products are designed for use in older people, even though the claim is that Abrysvo is fine for pregnant women (between 32 through 36 weeks gestation)1. 4.5% of the reports were made by people 40 years of age or less and 93% of these were made by women. Interestingly, 89% of these reports were filed in the context of the Abrysvo product. This will become important. There have been ~7,816,892 doses adminstered in the U.S. as of December 16, 2023.2 The reporting rate per million doses per month is shown in Figure 1.

What is perhaps most disturbing about this very early data in spite of the fact that the Abrysvo product is ‘designed’ for pregnant women, there is no long term (or short-term) safety data.3 The phase III clinical trial for Abrysvo (NCT04424316) was only just completed on October 27, 20234, so it is impossible to commit to safety claims relevant to pharmacovigilance, or to the health and well-being of the infants.
Pharmacovigilance is the way that we ‘see’ signals of harm in data that weren’t detected in the clinical trials. For example, if a bad result occurred during a clinical trial that is inconvenient with regard to ‘the vaccine program’ or to investors, then that bad result might get disappeared. Just sayin’. This happens. And it’s SHOCKING.
VAERS is a functioning pharmacovigilance tool. It is telling us now that 137 WOMEN (not people) reported being exposed to RSV vaccines during pregnancy (MedDRA code: “Exposure during pregnancy”) and of these women, 20% experienced pre-term labor or birth, unterine contractions with or without hemorrhaging or an emergency Caesarean section. 89% of these reports were in the context of the Abrysvo product and not the Arexvy - approved for people 60+ as previously mentioned.
VAERS ID 2718314 reported pre-term labor and delivery within 24 hours of vaccine administration. Her cramping and bleeding started 5 hours after injection.
2 women reported spontaneous abortions.
It is important that we pay attention to these signals. 20% pre-term births sounds high to me in the context of a vaccine. 33% of these pre-term births occurred within 24 hours of administration of the vaccine. Sound like causation to you? It might to Sir Bradford Hill. In addition, there is no follow-up and therefore we cannot know if these babies fared well or not. Let’s pray they did. I don’t feel optimistic however, since the mother is likely to inject baby at birth with HepB.
The Arexvy product is the same as the Abrysvo product with regard to “Warnings”.5 The only real difference between them is that the former is designed to ‘protect against’ both RSV A and B, and it is not adjuvanted like Arexvy is.
Here are the side effects as listed on Drugs.com for Arexvy and Abrysvo.
Low birth weight and jaundice also occurred at a higher rate in infants of pregnant recipients of Abrysvo versus placebo. Prescribing information for pregnant women will include a warning about a "numerical imbalance" in preterm births in Abrysvo recipients: 5.7 percent versus 4.7 percent in those who received placebo but this data is not sufficient enough to establish that the vaccine can cause preterm births, but provides a warning.
Low birth weight is associated with early or pre-term birth. I wonder if there’s a connection? I love how they put “numerical imbalance” in air quotes in the context of the pre-term birth warning. It’s like: yeah, our vaccine does show an association with pre-term births (1% higher than placebo) but our data is too weak and sparse to be worried about that as a problem in real life. Just take it! Whaaaat? It’s fine!
Really? REALLY?
Remember this? Last time I checked, foetuses were younger than 2 year-olds and my guess would be that the risks would apply to both. Although, I am not a medical doctor, I think it’s pretty clear that even in the pre-phamacogivilance data era, a signal would clearly arise. It did, according to the above.
Not good for animals = not good for babies or foetuses. I wonder if the foetuses delivered in the context of the women vaccinated as part of the NCT04424316 trial have been monitored for enhanced respiratory disease, as part of the original study design?
I am going to make a morbid bet that reports for babies are going to start coming into VAERS - complete with respiratory involvement. The CDC will likely just blame it on some new variant of RSV. God help us.
https://www.drugs.com/medical-answers/what-difference-between-arexvy-abrysvo-3574041/#
https://www.cdc.gov/vaccines/imz-managers/coverage/rsvvaxview/adult-vaccinations-administered.html
https://clinicaltrials.gov/study/NCT04424316
https://www.cdc.gov/vaccines/vpd/rsv/hcp/pregnant-people-faqs.html - the CDC still believes that ‘people’ can get pregnant
https://www.drugs.com/medical-answers/what-difference-between-arexvy-abrysvo-3574041/#






Vaccines should not be given to pregnant women full stop!.
In the last few months, I've had two adverse outcomes for pregnant women with the Covid modRNA treatment
1. Vaccinated antenatally 32/40 - severe pre-eclampsia and premature birth 34/40. Mother ongoing hypertension and kidney damage (microalbuminuria)
2. Vaccinated 3w postpartum. Breastfeeding. Developed breast blister at 4w. Baby developed viral meningitis at 5w, diagnosed as parechovirus
I think the immune response with these modRNA poisons is so hyper-focussed on one thing (eg producing spike protein for SARS-COV2) that they completely disrupt the normal functioning of the immune system. And the LNPs are inflammatory which can't be good for pregnant women.