Recipe for disaster: A dash of spike, a pinch of nurpy brain cells makes for an amyloid-β omelette that you're sure to forget about!
Because of neurodegeneration... Too early?
Spike protein loves your brain cells and amyloids love to party with spike.
A paper has recently (July 4, 2025) been published in Science Advances entitled: “SARS-CoV-2 induces Alzheimer’s disease–related amyloid-β pathology in ex vivo human retinal explants and retinal organoids”.1
The authors found that when they created human-derived tissues and introduced the spike protein from SARS-2, that the result was amyloid-β aggregation. Just to remind you all, that’s associated with Alzheimer’s disease, and amyloidogenic peptides are in the spike protein. The spike protein that was made in a lab. I have documented this copiously.
These results suggest that Spike 1 (S1) protein, during infection with SARS-CoV-2, can induce amyloid-β aggregation, which may be associated with the neurological symptoms experienced in COVID-19.
There’s no “may” about it: this is definitive.
We actually don’t quite know what causes Alzheimer’s disease, but there’s increasing evidence for a theory called the amyloid-β antimicrobial hypothesis. This idea suggests that amyloid-β, a protein that forms plaques in the brains of people with Alzheimer’s, might be produced when harmful microbes, like bacteria or viruses, are present. These amyloid-β clumps could be the body’s way of trapping and fighting off these microbes as part of its natural defense system.
More viruses/pathogenic material = more amyloid plaques = more neurodegeneration.
Amyloid-β is a small peptide, typically 36-43 amino acids long, that is thought to be associated with Alzheimer’s disease. It is produced when a larger protein, called amyloid precursor protein (APP) is cleaved by enzymes (specifically beta-secretase and gamma-secretase) in the brain. Amyloid-β is a normal product of cellular metabolism, but in Alzheimer’s disease, it can mis-fold and aggregate into insoluble fibrils, forming sticky plaques in the brain that disrupt nerve cell function and contribute to cognitive decline. The key difference between a normal brain and a plaquey brain is the clearance rate versus the accumulation rate: if the latter out paces the former: not good.
Amyloid-β may also play roles in synaptic activity and brain development and its precursor, APP, is involved in cell signaling and neuronal health.
It is important for me to point out (you’ll find out why soon or already know) that amyloids refer to a broader class of proteins or peptides that share a common characteristic: they misfold into a beta-sheet-rich structure and form insoluble, fibrous aggregates called amyloid fibrils. These fibrils have a distinct cross-beta sheet structure, which makes them resistant to degradation and prone to deposition in tissues. All kinds of tissues including the brain.
As already mentioned, amyloid-β is a particular amyloid derived from APP, a transmembrane protein found in many cells, particularly neurons. This is why when things go awry with amyloid-β functionality, neurological symptoms ensue. Other types of specific amyloids include:
Serum Amyloid A (SAA): An acute-phase protein produced in the liver, associated with systemic amyloidosis (AA amyloidosis) in chronic inflammatory conditions like rheumatoid arthritis.
Transthyretin (TTR): A transport protein that can misfold and form amyloid deposits in transthyretin amyloidosis (ATTR), affecting organs like the heart and nerves.
Islet Amyloid Polypeptide (IAPP or Amylin): A peptide hormone co-secreted with insulin in the pancreas, linked to amyloid deposits in type 2 diabetes.
Prion Protein (PrP): A misfolded protein causing prion diseases like Creutzfeldt-Jakob disease, which forms amyloid-like aggregates in the brain.
Location, location, location.
All amyloids share the beta-sheet fibril structure, but the amino acid sequences of amyloid-β and other amyloid proteins are distinct, leading to differences in how they aggregate, their toxicity, and their interactions with tissues. From a bio/nanotech point of view, amyloids are actually exploited for their robust fibril-forming properties, and can be engineered for drug delivery or tissue scaffolds.2
In this study, they used ‘electrophysiologically active ex-vivo human retinas from short-interval autopsies and in an accelerated aging human retinal organoid model’ in order to determine if spike protein induces amyloid-β aggregation to cause neurological symptoms.
The authors correctly point out that “there is a need to understand the aging central nervous system’s (CNS) response to viral infection”. I couldn’t agree more. But I would add amyloidogenic peptides delivered via lipid nanoparticles to this CNS response list.
It has been previously found that SARS-CoV-2 spike protein up-regulates amyloid-β through modulating γ-secretase3, but it must be taken into account that the spike protein actually contains amyloidogenic peptides itself. [2]4 In addition to altering amyloidogenic enzymatic processing via γ-secretase modulation, it very likely also contributes to an abundance of amyloids thus adding fuel to the fire, so to say, via aggregation and alteration of normal pathways via binding.
Just think about the enormous amount of spike protein being produced following injection with the modified mRNA COVID shots from Pfizer and Moderna, and then think about the amount following repeated re-injection, and then think of all the studies continuing to show that people are still making spike protein years out from their last shot. Can you say, integration? All I can say is, I am glad it’s not everyone.
Increased amyloid-β in patients with COVID-19 may be associated with compromised memory and learning processes that can result in neurological impairment.
You can say that again. There are hundreds of thousands of reports of neurological impairment in VAERS alone.
I would also say that any amyloid aggregation in the brain would lead to neurological impairment. If the amyloidogenic peptides are identical or similar to amyloid-β, they could integrate into existing Aβ plaques or seed new ones, increasing aggregation. If they are different (ie: resembling islet amyloid polypeptide or prion protein), they might form distinct amyloid aggregates or interact with other brain proteins, potentially causing different pathological effects. If introduced amyloidogenic peptides formed oligomers (repeating units), they could disrupt neuronal function, trigger inflammation, or exacerbate existing pathology, even if they didn’t form large plaques.
In order to visualize and quantify amyloid-β plaques in human retinas, they used a turmeric analog to stain for amyloid-β in the tissue. Is there anything turmeric isn’t good for? I had to mention this.
To show that the human retinas from postmortem donors with Alzheimer’s disease contained amyloid-β, we assessed rapid autopsy retinas from controls (n = 4) and Alzheimer’s disease (n = 3) for the presence of extracellular plaque formation using the small-molecule curcumin analog CRANAD-28 (34–37). CRANAD-28 does not require fixation, allowing for the detection, visualization, and quantification of amyloid-β plaques in human retinas.
So their resultant preliminary data showed amyloid-β deposits and neuropathology in retinas taken from deceased individuals that had Alzheimer’s disease.
The main meat of the paper comes from when the authors generated human retinal organoids by super-charging pluripotent stem cells. This is no small feat. After 2 months of work, they confirmed the presence of neurons in their organoids with neuronal markers. To ensure that they were decently recapitulating Alzheimer’s amyloid-producing disease features in their diseased organoids, they used both immunofluorescence and ELISA tests and found elevated levels of amyloid-β in their fancy Alzheimer’s disease retinal organoids as compared to the controls.
So their model worked.
These results show the presence of excitable retinal ganglion cells in the retinal organoids and functional human neurons.
When they checked retinal ganglion cells and macroglia, they saw high expression of Neuropilin-1 (NRP1), meaning that SARS-CoV-2 spike protein has affinity for these two cell types because of NRP1. Now, NRP1 is really important in the spike (particularly S1) context because it’s a co-receptor for cell binding and entry. This interaction has been linked to amyloid-β accumulation and is marked by spike presence. [1]5 So in cells like macroglia, who have high NRP1 expression, they are targets for ‘infection’ or at least binding by spike (S1).
It’s absolutely clear from the figure below that macroglia are the favorite.
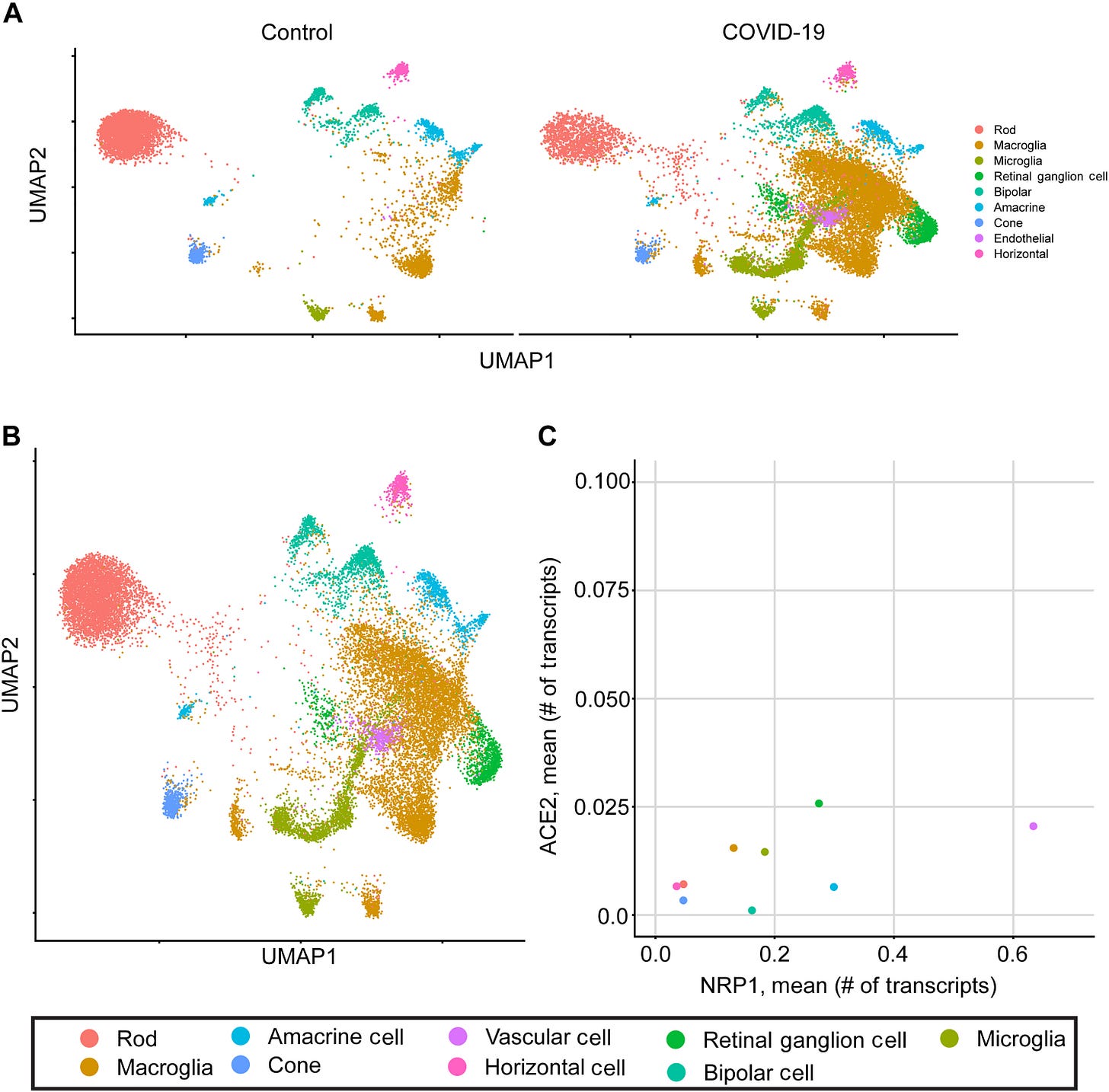
They also found that by blocking NRP1, they could reverse the effect of aggregation of amyloid-β.
Blocking NRP1 can decrease the amyloid-β deposition in human retinas following SARS-CoV-2 Spike 1 treatment.
The authors write:
Once amyloid-β is produced and secreted, it binds to S1 protein with high molecular affinity, which leads to amyloid-β aggregation and encapsulation as a defensive mechanism.
Sure. But what if spike is the amyloid?6
And they conclude:
In conclusion, these data suggest that COVID-19 infection, through the SARS-CoV-2 S1 protein, leads to Alzheimer’s disease–related pathology.
There is no reason to believe that if the S1 protein from SARS-2 is doing this that the spike protein produced by the cells of the COVID-19 injectable product recipients would not also do this, especially since we also know that the RNA templates were likely not leading to translation of full length spike protein.
The findings in this paper are alarming, and this work should have been done long before any prototype experimental gene-based therapy - to include a nucleoside-modified mRNA template of that very same spike protein (however 2P modified to yield pre-fusion version) - was injected into a signal human being! Let’s not forgot that these experimental and now provably dangerous products were mandated under penalty of job loss, other threats (remember when Sleepy Joe said “We’ve been patient, but our patience is wearing thin”) and loss of friends and family, and put onto the bloody childhood vaccination schedule in the United States. They expected babies - infants! - to get these things: 3 before 18 months, unless I stand corrected.
This last abhorrent move by regulators/FDA has since been reversed by Robert F. Kennedy Jr., but this gene-based LNP platform - especially when it involves dangerous mRNA templates encoding proteins that are seriously detrimental to human health - needs to be TAKEN OFF THE MARKET.
TODAY.
Sean J. Miller et al., SARS-CoV-2 induces Alzheimer’s disease–related amyloid-β pathology in ex vivo human retinal explants and retinal organoids.Sci. Adv.11, eads5006(2025).DOI:10.1126/sciadv.ads5006
Valeria Castelletto and Ian W. Hamley. Amyloid and Hydrogel Formation of a Peptide Sequence from a Coronavirus Spike Protein. ACS Nano 2022 16 (2), 1857-1867. DOI: 10.1021/acsnano.1c10658
G. Ma, D. F. Zhang, Q. C. Zou, X. Xie, L. Xu, X. L. Feng, X. Li, J. B. Han, D. Yu, Z. H. Deng, W. Qu, J. Long, M. H. Li, Y. G. Yao, J. Zeng, SARS-CoV-2 Spike protein S2 subunit modulates γ-secretase and enhances amyloid-β production in COVID-19 neuropathy. Cell Discov. 8, 99 (2022)
Charnley, M., Islam, S., Bindra, G.K. et al. Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: potential implications for neurological symptoms in COVID-19. Nat Commun 13, 3387 (2022). https://doi.org/10.1038/s41467-022-30932-1
Chen Y, Zhang X, Li J, et al. SARS-CoV-2 spike protein S1 subunit induces neuroinflammatory response and amyloid-β pathology through neuropilin-1 receptor. Aging Dis. 2024;15(4):1473-1485. doi:10.14336/AD.2024.0731
Charnley, M., Islam, S., Bindra, G.K. et al. Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: potential implications for neurological symptoms in COVID-19. Nat Commun 13, 3387 (2022). https://doi.org/10.1038/s41467-022-30932-1





Neurodegenerario?
Neuroinflammation?
Color me shocked. 🙄
That LNP delivery system really gets into the central nervous system effectively.
Safe and effective. For chronic disease causation and depopulation for fun and profit.
Very good Jessica, thank you. It's exactly like a slow-moving Trojan Horse. Sleepy Joe also stated those who did not get the v=a=x would be sorry---a THREAT to the people.
To back your article Michael Nehls, MD, PhD, author of The Indoctrinated Brain, states:
The major driver of this brain-damaging neuroinflammation is the S1
subunit of the spike protein, which can enter the brain very efficiently
across the blood-brain barrier after being shed or cleaved at the furin
cleavage site.
For example, mice injected with the S1 subunit exhibited HIGHLY
STRESSED BEHAVIOR and elevated levels of proinflammatory mediators
such as TNF-α and IL-6, which are associated with CEREBRAL VASCULAR
DAMAGE.81
In addition, the S1 subunit was found to interact with the PRION
protein, causing it to fold abnormally and form toxic aggregates that can
cause CEREBRAL PRION DISEASE.82
The S1 subunit also binds to β-amyloid. This endogenous peptide is
released by the hippocampus as a monomer during memory formation
and helps prevent new memories from overwriting previous ones.
However, when these monomers [similar to the prion protein] aggregate
to form oligomers, neuronal synapses and thus HIPPOCAMPAL MEMORIES
ARE NOT PROTECTED, but DESTROYED. The OLIGOMERS, which are toxic
to nerve cells, are therefore SUSPECTED OF ACCERLERATING the
ALZHEIMER's process once it has begun.83 However, binding of the S1
subunit of SARS-CoV-2 to β-amyloid ALSO ACCELERATES VIRAL INFECTION
or VIRUS ENTRY into somatic cells, increasing the release of proinflammatory
messengers, which could also be a reason for hippocampal damage and
increased Alzheimer’s risk.84 While the interaction of spike protein with
aggregation-prone proteins such as prion protein or beta-amyloid in the
brain may lead to neurodegeneration, ANOTHER POSSIBLE NEUROTIXIC
mechanism is the cross-reaction of antispike protein antibodies with the
antigens of neuronal tissue. It is therefore not surprising that in addition to
DEMENTIA and other brain abnormalities after COVID-19, cases of rapidly
progressive DEMENTIA AND AUTOIMMUNE ENCEPHALITIS have already been
reported after spiking.85