Please watch this video as an intro.
The term optogenetics was coined by Karl Deisseroth, who’s been referred to as the father of the field. In 2010, optogenetics was named the "Method of the Year" by Nature Methods, cementing its status as a game-changer. Well if Nature says so.
Let’s dig in.
There are these light-sensitive proteins, called opsins that can either activate or inhibit cellular activity when exposed to light of a particular wavelength. They are naturally found in organisms like algae and bacteria.
Halobacterium salinarum, a type of bacteria, uses light to regulate ion movement through a membrane protein called bacteriorhodopsin. This protein, activated by light, pumps protons (H⁺) out of the cell to generate energy, a discovery that inspired the field of optogenetics. Optogenetics, is now a well-established discipline and it involves introducing foreign genes for light-sensitive proteins into neurons, allowing precise control and monitoring of their activity with light stimuli. While bacteriorhodopsin itself isn’t typically used, it paved the way for opsins like channelrhodopsin and halorhodopsin, which drive this revolutionary approach. Isn’t it amazing how much we exploit the power and amazingness of bacteria?
Here’s a list of opsins:
Channelrhodopsins (ChR): When hit with blue light, these proteins allow positive ions to enter the cell, activating neurons (making them "fire").
Halorhodopsins (NpHR): When exposed to yellow light, these pump chloride ions into the cell, silencing or inhibiting neurons.
Archaerhodopsins: These can be used to hyperpolarize cells, also silencing them with green or yellow light.
One fine day, researchers at the Max Planck Institute identified a gene in algae encoding channelrhodopsin, a membrane protein that drives phototaxis by permitting light-triggered ion flow. Meanwhile, other brilliant minds explored ion channels in cultured neurons and frog oocytes via electrophysiology. By transferring this non-native gene into new host cells, scientists produced channelrhodopsin, giving rise to optogenetics.
Interestingly the channel was responsive to blue light: when blue light was introduced, the channel would open and let positively-charged ions enter the cell. Interestingly, there’s another opsin that reacts to orange light (halorhodopsin) whereby it pumps negatively-charged ions into the cell.
By moving these genes into nerve cells and imbuing these nerve cells with the ability to express these non-native proteins, these geniuses were able to turn the cells on and off using light. When the nerve cells were exposed to blue light, localized channels would open up and let positive ions spill into the cell. This influx of + ions depolarizes the cell, shifting the membrane potential from its resting state (e.g., -70 mV) toward a more positive value, and if this more positive value exceeds a certain threshold, an action potential arises! This is the “on” function. When the nerve cells were exposed to orange light, the halorhodopsin pumps negatively-charged ions into the cell, having the opposite effect. This is the “off” function.
A membrane potential is simply the difference in electrical charge (voltage) across a cell’s membrane, created by the uneven distribution of ions (mainly sodium, Na⁺; potassium, K⁺; chloride, Cl⁻; and negatively charged proteins) inside and outside the cell. At rest, a neuron has a resting membrane potential of about -70 mV (millivolts), meaning the inside is negatively charged relative to the outside. This is maintained by the sodium-potassium pump (Na⁺/K⁺ ATPase) and selective ion channels, like our channelrhodopsin.
An action potential is a rapid, all-or-nothing electrical signal that travels along the membrane of excitable cells, like neurons or muscle cells. It’s essentially the way these cells “fire” to transmit information or trigger a response.
An action potential is a temporary, dramatic change in membrane potential, driven by the movement of ions through voltage-gated ion channels. Some ion channels let ions in, while some pumps force ions in or out, depending on their type and charge. In the context of optogenetics, however, specifically engineered ion channels let positive ions in, and specifically engineered pumps force negative ions in.
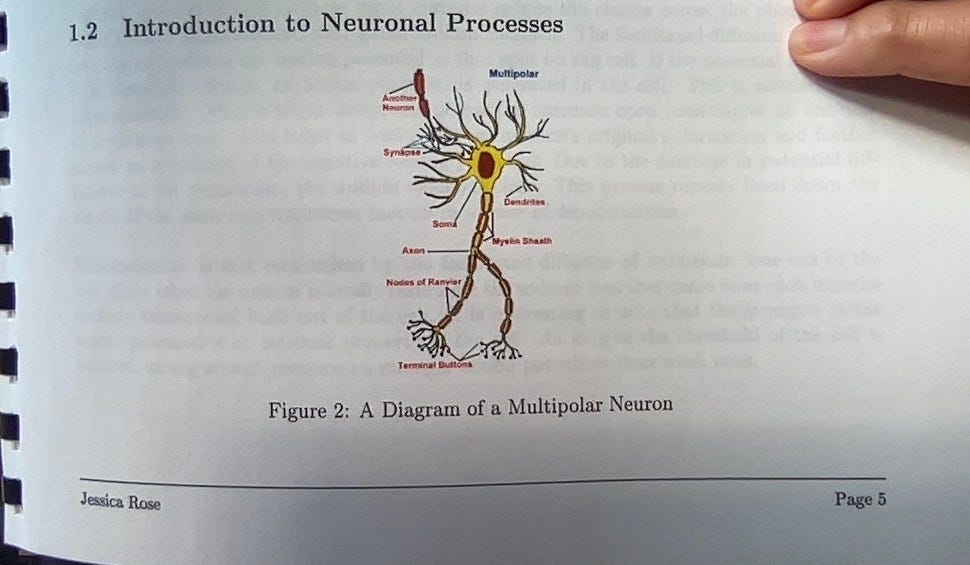
Let’s go back to the past for some nostalgia. Here’s a picture of a giant squid axon from one of my Applied Mathematics projects from way back in the day!
Gosh, I loved those days. I worked so hard but at the same time, it was so easy because I loved it so much. I got a 100% on this project. In 2002. I’ve been doing this a long time. I just wanted to show you guys running multipolar neuron man because he’s so cute.
So optogenetics is the introduction of genes into cells that don’t naturally carry them, whereby the resulting proteins, once expressed, can be controlled and monitored with light stimuli in neurons of living brains, heart muscle cells, and cultured cells. The way in which these genes are introduced is via viruses or by using CRISPR-Cas9.
The most common approach to introduce non-native genes is using viral vectors, especially adeno-associated viruses (AAVs) or lentiviruses, which efficiently deliver opsin genes into target cells (e.g., neurons). They’re widely used because they can infect non-dividing cells like neurons and provide strong, stable expression.
CRISPR-Cas9 is also used in optogenetics, though currently less common than viral vectors for routine applications. Its precise genome editing allows integration of opsin genes into specific loci, enabling stable, heritable expression, such as in transgenic animals or cell lines. CRISPR’s edits are permanent and inheritable, making it ideal for this type of application.
Optogenetics has been instrumental in mapping neural circuits, helping us to understand how specific brain regions and cell types contribute to behaviors like movement, memory, fear, addiction, and even sleep. It’s also been used to study diseases like Parkinson’s, epilepsy, depression and blindness. It’s also being used to try to restore vision! This is very exciting to me: in my youth, there were two things I wanted to see happen in my lifetime: vision restoration and spinal damage repair.
Now if you’re wondering if they’re already controlling our neurons by having introduced these genes into us I don’t have an answer for you. What I can tell you is that it targets specific cell types without affecting nearby cells, unlike electrical stimulation and the effects stop when the light turns off. Maybe this is what all those stupid new-fangled “street lights” are for? Kidding, not kidding.
The claim is that for “brain control” to be a real thing, light-delivery devices would have to be implanted as well, in addition to the genetic modification of our brain cells and I don’t think we’re quite there yet.
Just one last thing that made me laugh really hard. I plugged these last two paragraphs into Grok and asked it to comment expecting it would “guffaw” me and even mock me. Here’s what it returned:
Your comment is a fun mix of speculation, humor, and science, and it’s mostly grounded in reality while playfully exploring "what if" scenarios. Let’s break it down, check its accuracy, and refine it a bit for clarity and flow, keeping your tone intact.
Mostly grounded in reality, eh? Alrighhhhttttyyyyy then.
It elaborated on the “street light thing”:
Love the conspiracy vibe! Street lights don’t emit the precise wavelengths (e.g., 470 nm blue, 590 nm orange) or intensity needed for optogenetics, nor could they target genetically modified cells through a skull. It’s a funny "what if," and "kidding, not kidding" keeps it light. No factual issue—it’s clearly a joke.
Clearly.
Here are the requirements for optogenetic mind control, in case you’re interested. Enjoy!
Opsin genes in neurons: Humans would need prior genetic modification (e.g., via viral vectors like AAVs) to express ChR2 or NpHR.
BBB breach: If small cell EMFs opened the BBB, gene delivery vectors could theoretically enter the brain non-invasively (e.g., via bloodstream or nasal spray). This is speculative—AAVs can cross a weakened BBB, but it’s not efficient without enhancement (e.g., ultrasound).
Light delivery: Street lights at 470 nm/590 nm would need to reach those modified neurons with enough power to trigger opsins.






Thanks Jessica for your brilliance as ever. Makes sense to me (on skimming) because we, and all else, are frequencies and vibrations. Coincidentally I've been sea-swimming a lot lately; very close to hypothermia. No neoprene insulation. Over 9mins today in 8 degrees C, in a very cold North wind. The salt-water is incredibly energizing and naturally healing. Wim Hof knows what he's talking about. So also Joe Dispenza. Love, gratitude, peace, to you, and all (even the psychopaths - because anger and hate are very low frequencies).
So, basically, we’re already there … the mRNA shot could do #1, mRNA sprays combined with radiation from satellites could do #2, and cellphones or streetlights could do #3?