Do the LNPs adsorb apoE for cell/tissue-specific delivery of contents?
What this means and why it matters
YES. Says I.
This means that the LNPs - yes, the very same ones that carry the modified mRNA and plasmid-derived DNA that were injected into billions of people - are very likely targeted to the liver, and other low density lipoprotein receptor (LDLR)-expressing tissues. Remember this pharmacokinetic study from Japan? Look at the mean total lipid concentration in the liver after 8 hours: 26.5 ug lipid equivalent/g. And 16.2% of the total administered dose was in the liver after 48 hours.
That’s comparable to the 24.6% measured at the injection site. Maybe, in addition to the LNPs having alleged neutral charge12, it is in fact the apoE targeting these things to the liver. Before I start, the mechanism of action here is receptor-mediated binding. The tissues/cells with the cognate receptors for apoE will bind apoE, and anything coated with it.
It is not just the liver that contains apoE receptors. I will return to this point.
It matters because it seems highly unlikely to me that neither the researchers, developers nor manufacturers of these cholesterol-rich fat bubble nano carriers (LNPs) did not anticipate that these LNPs would be preferentially targeted to LDLR-expressing cells (and tissues).
This is important, in fact: KEY, because we were explicitly told that these things would stay at the injection site.
For the record: there are 3 types of apoE - 2 (5% of population), 3 and 4 (25% of the population). Having apoE4 type contributes to Alzheimer’s risk. ApoE4 has arginines at positions 112 and 158. ApoE2 is protective against Alzheimer’s and has cysteines at positions 112 and 158. ApoE3 has a cysteine at position 112 and an arginine at position 158.
Since the apoE2 version is the best cholesterol carrier/amyloid scavenger of the 3, I found myself thinking of the other 2 versions as mutations of this ‘best’ version. Well, best in terms of Alzheimer’s.
Here’s the crystal structure of apoE2 to give you an idea of where the ‘mutations’ lie to manifest in these wildly minor, but wildly physiologically major differences. Arginines are big, by the way. So if you swap out a cysteine for an arginine, you’re likely going to affect the conformation of the protein, not just what it typically binds. I made a demo mutation at position 158 for you to see the differences in space that arginines and cysteines occupy.
What a difference a single amino acid can make. God. Nature.
Let’s back up the bus a bit and talk about what some of these acronyms are.
On lipoproteins and apolipoprotein E (apoE)
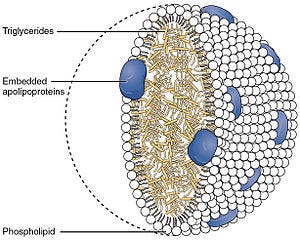
A lipoprotein in general, is a cholesterol and triglyceride carrier with special properties. (I had no idea how vital cholesterol was to life before researching this.) Lipoproteins have the task of transporting fats in water (as in blood plasma and extracellular fluids) so they are specially designed to enable this with hydrophilic (water-loving) portions oriented outward toward the surrounding water (or blood plasma), and lipophilic (fat loving) portions oriented inward toward the lipid center.3
Here’s the official definition of a lipoprotein:
A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid (also known as fat) molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center.
Of these lipoproteins, there are special kinds called apolipoproteins. These are embedded in the outer shell of a lipoprotein, as shown below, acting to stabilize the lipoprotein complex and also imbuing it with function. I love that word. Imbue.
Of these apolipoproteins is apolipoprotein E or apoE for short, and this particular protein is really important in fat metabolism in mammals. ApoE is also critical to normal brain functioning as it is the principal carrier of cholesterol in the brain and is required to carry it from astrocytes to neurons.4
One of the most ubiquitous protein fat bubbles essential to fat metabolism of ingested fats is called a chylomicron. A chylomicron is a by-product of the ingestion of fats whereby fats go through the thoracic duct prior to ultimately being trafficked to the liver for final metabolism. On the way via the intestine, the fats get distributed to muscle tissues, adipose tissues - all sorts of places that need fats for energy or storage - by means of these special protein fat bubbles called chylomicrons that are embedded with apolipoproteins.

As they finish the job of delivering fats from the intestines to the body, they get depleted, and as such, become chylomicron remnants. These chylomicron remnants - besides being smaller - are embedded with apoE. My guess would be that as the diameter of the particle decreases with depletion of contents, the surface area contents would become more concentrated. These chylomicron remnants then get trafficked to the liver where apoE receptors, also known as low density lipoprotein receptors (LDLRs), are plentifully expressed. This is by design. The chylomicron remnants are purposefully trafficked to the liver for further breakdown (metabolism) of this particle, and production of very low density lipoproteins (VLDLs).5 And the cycle continues…
While circulating in blood, chylomicrons exchange components with high-density lipoproteins (HDL). The HDL donates apolipoprotein C-II (APOC2) and apolipoprotein E (APOE) to the nascent chylomicron and, thus, converts it to a mature chylomicron (often referred to simply as "chylomicron"). APOC2 is the coenzyme for lipoprotein lipase (LPL) activity.6
Now onto the nitty gritty.
The authors of a peer-reviewed publication entitled: “The role of lipid components in lipid nanoparticles for vaccines and gene therapy”, make the following statement in reference to exploiting our biology for drug targeting strategies:
Consequently, the apoE adsorption on LNPs turn them into biomimicking remanent chylomicrons that are taken up by hepatocytes.7
Another paper was published in 2021 entitled: “Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles”, and they found that binding of apoE to the LNPs induced a lipid redistribution at both the shell and the core levels that impacted the LNP internal structure causing mRNA release.8 Since apoE is prevalent in the blood serum, this would indicate that LNPs may be subjected to discombobulation if apoE studded their surfaces, and site-specific trafficking and transfection by receptor-mediated endocytosis. Since this raises yet another huge research question, ie: what effect does this discombobulation have on a person’s physiology, let’s leave it on the back burner for a second. Or until my next article.
Getting back to the LNPs as biomimicking remanent chylomicrons, I think it’s also important as part of this story to bring up a drug called Onpattro. (Thank you to Maria Gutschi for bringing this to my attention.) Here’s a good article about it: “The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs”.9
In short, Onpattro is a lipid nanoparticle-based short interfering RNA (siRNA) drug that acts to inhibit the synthesis of the transthyretin (TTR) protein in the liver to treat polyneuropathies induced by hereditary transthyretin (ATTR) amyloidosis. Interestingly enough, I am no stranger to this type of amyloidosis having investigated it as one of the possible things that might go wrong if the liver was bombarded by LNPs and spike protein. You can hear me talk about that here (at around timestamp 27:00) as part of my National Citizen’s Inquiry testimony.
The reason I wanted to bring Onpattro into this story is because of the similarities between its design and the design of the COVID modified mRNA products. The only real difference between them is their contents. The Onpattro contains silencing RNA (siRNA) and the COVID products contain modified mRNA (modmRNA). Their LNP constituents are the same. They both utilize ionizable cationic lipids, PEG, helper lipids (DPSC) and cholesterol.
All the current FDA-approved LNP formulations contain four lipids: (1) an ionizable cationic lipid, helper lipids which include (2) 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and (3) cholesterol, and (4) a polyethylene glycol (PEG)-lipid conjugate. These constituents facilitate monodisperse nanoparticle formation, improve nanoparticle stability, enable efficient nucleic acid encapsulation, aid cellular uptake, and promote endosomal escape of nucleic acid cargo.10
The developers of Onpattro brilliantly exploited the fat metabolism system of humans to target the siRNA to the liver to inhibit the synthesis of a liver-derived protein by using this fat bubble as a carrier/apoE system. So my question is this:
Why wouldn’t the COVID LNPs be treated precisely the same way by the human body? The role of apoE is to bind to fat bubbles and bring them to the liver (or cells expressing LDLR), so why wouldn’t apoE adsorb to the COVID-19 product LNPs and bring them to the liver (and all LDLR-laden cells)?
I think they do.
The following slide shows the distribution of expression of LDLR in the human body from The Human Protein Atlas (HPA). It’s expressed in a lot of places in the human body. It is officially referred to as MALRD1 in the HPA.
One more thing I invite you to investigate is the relationship between PEG and apoE. PEG desorbs from the LNP upon administration. ApoE promotes its desorption.
I am anxious to publish this article because I am on a deadline for a slide deck. But it’s important as yet another striking piece of evidence that contradicts the “it stays at the injection site” edict that was so relentlessly, patronizingly and venomously spat at all of us for years. Not only is this not true, but this article provides strong evidence that at the very least, THE RESEARCHERS, DEVELOPERS OR MANUFACTURERS SHOULD HAVE KNOWN. It is far more likely, that they did know and didn’t care.
As usual, comments welcome.
Cheng, Q., Wei, T., Farbiak, L., Johnson, L. T., Dilliard, S. A., & Siegwart, D. J. (2020). Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nature Nanotechnology, 15(4), 313–320. doi:10.1038/s41565-020-0669-6
Kranz, L., Diken, M., Haas, H. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016). https://doi.org/10.1038/nature18300
https://en.wikipedia.org/wiki/Lipoprotein
Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc Natl Acad Sci U S A. 2021 Aug 17;118(33):e2102191118. doi: 10.1073/pnas.2102191118. PMID: 34385305; PMCID: PMC8379952.
Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997 Nov;38(11):2173-92. PMID: 9392416.
https://en.wikipedia.org/wiki/Chylomicron
Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022 Sep;188:114416. doi: 10.1016/j.addr.2022.114416. Epub 2022 Jul 3. PMID: 35787388; PMCID: PMC9250827
Federica Sebastiani, Marianna Yanez Arteta, Michael Lerche, Lionel Porcar, Christian Lang, Ryan A. Bragg, Charles S. Elmore, Venkata R. Krishnamurthy, Robert A. Russell, Tamim Darwish, Harald Pichler, Sarah Waldie, Martine Moulin, Michael Haertlein, V. Trevor Forsyth, Lennart Lindfors, and Marité Cárdenas. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles. ACS Nano 2021 15 (4), 6709-6722. DOI: 10.1021/acsnano.0c10064
Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope MJ, Madden TD, Mui BL, Semple SC, Tam YK, Ciufolini M, Witzigmann D, Kulkarni JA, van der Meel R, Cullis PR. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019 Dec;14(12):1084-1087. doi: 10.1038/s41565-019-0591-y. PMID: 31802031
Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022 Sep;188:114416. doi: 10.1016/j.addr.2022.114416. Epub 2022 Jul 3. PMID: 35787388; PMCID: PMC9250827









I lost my brother to Covid. He died alone, isolated, on a vent just after his 51st birthday. He thought he’d get iv antibiotics for bronchitis; and he knew not to agree to Covid protocols. He was alone, vulnerable and pressured. My sense is that the current race wars are purposely being fueled by the same power structures that produced the Covid mess so that research like yours is buried and our freedom of speech is further suppressed. It is very hard to stay positive. Thank you so much for your time, I am encouraged to know that you are giving this information more visibility.
Thank you so much, for all of the work you’ve done. Whenever I see you on a podcast... it eases my mind a tiny bit. But I think it is your laugh. I’ve lost mine over the last few years. I hope that you never lose yours. Because it is like sunshine with a happy face.
(Mental image, cartoon sunshine smiling over the world)
That’s a weird thing to tell someone. But I’ve learned to just tell them. Because they might not be here tomorrow if you change your mind.
So thank you for your laugh. And all of your hard work.