DNA found integrated in cancer cell line
Implications for people injected with the modified mRNA products, specifically with regard to cancer
Layperson’s summary
DNA integration associated with the modified mRNA shots is an issue. It is an issue because cancer. Let’s break it down like a nuclear membrane during mitosis. When cells divide, their nuclear membrane has to break down and be re-formed as part of DNA copying and distribution to daughter cells.1 Any foreign DNA that might have found its way into the cell could get packaged into the nucleus when the nuclear membranes reform. It could also get into the nucleus via transport by nuclear localization sequences like SV40.2
Getting foreign into nucleus: not a problem.
Double-stranded DNA breakage (DSB) and repair are ongoing processes in cells. The former can become problematic due to accumulation of reactive oxygen species (ROS), and exposure to irradiation (ionizing radiation) and other ‘chemical agents’. But as nature would have it, cells have amazing inherent mechanisms to repair these breaks in order to prevent cell death, and to prevent genomic rearrangements commonly found in cancers (mutations). Collectively, these are called the DNA damage response (DDR). If the DDR is impaired or not utilized: cancer.3 Basically, anything that messes with DNA repair = bad/cancer.4
N.B. Don’t forget that it was published that spike protein impairs DNA repair mechanisms.5 This paper was retracted by the overlords so you know it’s important.
I can imagine that a fragment of foreign DNA might find a cozy spot in the human genome by exploiting a DSB. Perhaps instead of the break getting repaired end-to-end, an extra piece gets spliced in?
Getting a small fragment of DNA into the genome: not a problem.
Background
Professor Ulrike Kämmerer (OB/Gyn) in Germany decided to add some drops of the Pfizer modified mRNA vial contents to some cancer cells. I guess she had access to these particular cells being an OB/Gyn, and had some science-y type questions about transfection-ability of these cells by LNPs, and also potential DNA product contaminants.
On OVCAR3 cell lines
One of the cell lines she used in her experiment is called OVCAR3. The cells from the OVCAR3 cell line (aka: a human ovarian carcinoma cell line) are epithelial cells taken from someone who had ovarian cancer, once upon a time. They are typically used to study ovarian cancer drug resistance. She used a technique called immunohistochemistry on the OVCAR3 cells following exposure, to seek out S1 spike protein. Figure 1 shows that she found S1 (as denoted in brown) when examining the Pfizer lot GH9715 following the first passage (re-seeding or ‘subculturing’ in new medium) of cells.

Just so you know, passaging is done to maintain cell health for experiments by refreshing their living environment and food source. Imagine the state you’d be in if you didn’t wash and eat fresh food regularly: you’d be crusty and clumpy, and probably not interested in performing well in an experiment. It’s also how you’d discern between cell and supernatant. One of the by-products of passaging cells is that random mutations can occur in the subsequent progeny, so a higher number of passages could mean a cell population less like the original population, depending on the cell type. In the case of our OVCAR3 cells, they were passaged twice, and it is important to note that studies have previously shown that the mutation rate of these cells by passage is low, in fact. It’s also been shown that they have high non-homologous end joining (NHEJ) activity: a DSB repair pathway.6 I will come back to this.
So, Dr. Kaemmerer found spike in the OVCAR3 cells. This means the cells got bueno transfected, ie: the LNPs got into the cells and dumped their cargo.
What happened after she did this, you ask? One of the things that happened is that Dr. Kaemmerer reached out to Kevin McKernan to ask him if he could do the DNA seeking part of the experiment. He’s all set-up to do so, and is one of the only independent genomics experts looking into these important questions. Let’s go to Kevin’s findings.
On qPCR and sequencing findings
It seems that the stow-away foreign DNA cargo got into the DNA of the OVCAR3 cells as well. Kevin used qPCR to measure the amount of DNA in the COVID-19 injectable product vials alone (cts as low as 12.72 found), and in the cells that were subjected to the vial contents in order to establish a baseline of DNA ‘contamination’ degree. He then sequenced these products. It’s expensive to ‘do’ sequencing, so it was important to define the qPCR positive products first, and then sequence those.
According to Kevin’s preliminary findings, not only did the DNA get into the genomes of the OVCAR3 cells, but the transfected cells appear to be involved in mutating specific bits of the injection DNA itself, which could only mean that it is being replicated. These mutations were found in origins of replication (ORIs) - including the SV40 ORI, and were only found in the injection material/OVCAR3 cell context, and not in the injection material alone context.
What does this mean you ask? Let’s first review cell division and more specifically, mitosis.
On cell division and mitosis and chromosomes
Cell division by mitosis is the partitioning of genetic material to daughter cells in eukaryotic somatic cells (somatic cells are non sperm ‘n’ egg cells). OVCAR3 cells are eukaryotic somatic cells with ovarian origin, and their doubling time is about 50 hours.78 The genetic material is DNA and all of it needs to be duplicated, and this is done with the help of special enzymes. Figure 2 shows condensed DNA as part of the mitotic part of the cell cycle that includes prophase, prometaphase, metaphase and anaphase. Can you guess why it needs to be condensed?

Eukaryotes accurately duplicate their DNA once prior to cell division: one DNA duplication event = 1 cell division. (Replication is the same as duplication is the same as copying, in this context.) The following is an excellent video explaining all about DNA replication.
Here’s a video showing real life cell division of kidney cells. Wow. The red things are the sister chromatids (packaged/condensed DNA).
The reason I bring up chromosomes is because you need to know what they are in order to understand a DNA integration event by location within the chromosome, because Kevin found evidence of integration events within 2 specific chromosomes: chromosomes 9 and 12 (chr9 and chr12, respectively).
We humans have 46 chromosomes: 23 from mom and 23 from dad. Chromosomes are condensed forms of DNA (chromatin). It is necessary to condense the DNA prior to cell division because we have about 2 meters of DNA in every single cell! We normally see our DNA represented this way - as these little X-shaped guys - because they become more visible under a microscope when they’re condensed like this. The DNA in a cell that’s not dividing is more ‘noodle-y’, and less ‘X-y’. The following video is really good at explaining the world of chromosomes and karyotyping. And it’s nice, because they actually acknowledge that their cartoons are not scientific and don’t represent reality, unlike the CDC.
The following image shows chromosomes during metaphase. The centromere is the pinched part that joins the two sister chromatids to ensure equal partition of the genetic material.

The following gif shows mitosis in stem cells.

An important thing to understand is that mistakes can be made during DNA replication - like the ones made during passaging, even with the brilliant proofreading mechanisms to ensure perfect duplication every single time. Here’s a word on cancer in the context of bad copying and epigenetic factors. We are cancering all the time. Cancer is just the by-product of uncontrolled cell division in multicellular organisms, like us. A mutation in a gene that controls cell proliferation could lead to a cell becoming cancerous, for example. Our amazing bodies have built-in mechanisms to prevent and remedy cancerous cells, and considering all of the carcinogens in our living environments, they’re pretty good at both prevention and remediation, aren’t they! Think back to DSB repair. If a mutation or set of mutations arises from bad copying, then this might give the cancer cell a selective advantage. This is what might subsequently lead to invasive metastatic carcinomas.
This whole cancer thing is very chicken and eggy to me. I am still thinking about it. Does the cancer arise from the mutation or do the mutations arise from the cancer. Or both? Both, I’d say.
Errors in copying can lead to cancer9, and epigenetic factors can lead to cancer10. It is also important to acknowledge with regard to copying, that we’re not only dealing with bad copying potentially resulting in different genes, but differences in the ways and magnitudes that these genes, altered or not, are expressed. But let’s leave that on the back burner for now. Let’s try to link this to integration events and what Kevin found.
On integration events
An integration event can occur when a cell is dividing, or by human cell repair mechanisms when there is breakage in the DNA. The DNA would have to be uncoiled in order for a fragment of DNA to be spliced in at some specific location, in any case. Remember I mentioned NHEJ? This is one method of joining DNA bits together, say, once a DSB has occurred.
Another method of dsDNA repair mentioned by Kevin as part of his findings is called micro-homology mediated end joining (MMEJ). Figure 4 shows the difference between NHEJ, MMEJ and homologous recombination (HR). As previously mentioned, NHEJ has been shown to be more common in our OVCAR3 cells, so it is therefore even more telling that Kevin found ‘hallmarks’ of MMEJ in one of the detected integration events in the OVCAR3 genome. Interestingly, MMEJ is swapped in when homologous recombination (HR) (another way to join DNA - think meiosis) is deficient, and half of all ovarian cancers are deficient in homologous recombination (HR). MMEJ “always involves insertions or deletions, so it is a mutagenic pathway”. “Cells with increased MMEJ may have higher genomic instability and a predisposition towards cancer development.”1112
Let’s imagine that an integration event happened in a human cell following transfection with the Pfizer LNP-modified mRNA product. This is what we might refer to as illegitimate DNA integration: a homology-independent means to incorporate DNA.13 According to this study14, illegitimate DNA integration can happen more readily when the foreign DNA borrows some ‘natural’ (homologous) DNA and attaches it to on side - kind of like having an oven mitt on your left hand to grab your Le Creuset from the oven: it would be more likely that you would grab the left handle and hold onto it if you weren’t getting burned by it. I hope I didn’t go too off-the-handle here. Hardy har har.

For context, since we’re talking about integration of foreign genetic material into organism-specific DNAs, it is also important for everyone to remember that many human DNA- and RNA-containing viruses are capable of integrating their genomes into the genomes of their host cells. We’re all familiar with some of these viruses. HIV15 and Hepatitis B virus (HBV)16 are two examples. The point is that these integration mechanisms exist in nature. They can be absconded, exploited and in some cases, for lack of a better word, abused.
I’ve mentioned that you guys should read up on the CRISPR/cas system, and I will mention it again here because it’s the system that most bacteria and archaea use as an immune defense against viruses, phages and other genetic elements.17 They use this system, as a genetic material integration system, to splice in foreign bacteriophage DNA to protect themselves against subsequent challenge from these, and similar bacteriophages. Nature is wild, isn’t it!?
Seeing as how we’re talking about gene therapy/integration/dsDNA repair mechanisms, it’s worth mentioning mice with glowing eyes too.
Transgenic animal models are used to study the function of specific genes and disease aetiologies, aka: the pathophysiological roles of genes. A good example of one such model is the transgenic mouse model where mice have non-native genes (DNA chunks) introduced, either via retroviral infection of mouse embryos, introduction of foreign DNA into fertilized oocytes, or by targeted manipulation of mouse embryonic stem cells18, to manifest specific characteristics, like green glowy eyes and ears. Imagine seeing these little guys running around your place at night!

Nature, and we, are very good at manipulating DNA sequences for protection, survival, reproduction and … other stuff.
On reparsing Kevin’s findings
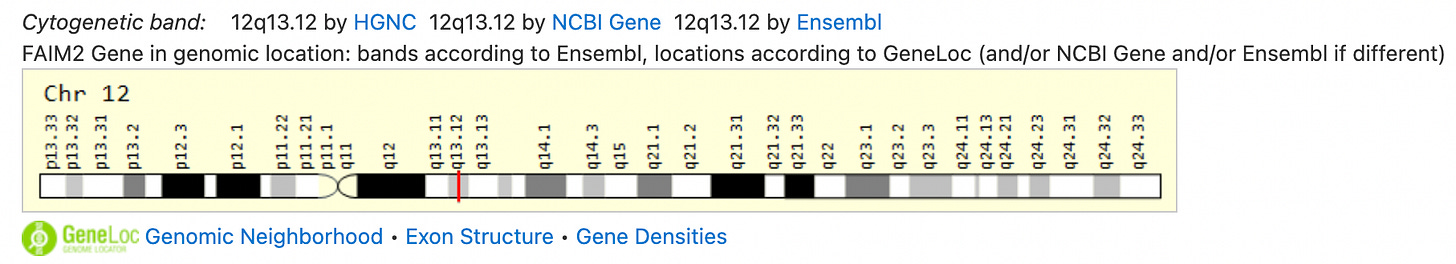
A nice reparsing of Kevin’s work was done by Mao Arakawa (Okudo Hirokushi) and you can read that here in Japanese (I love how the translation for Deep Sequencing is Deep Sea Quencing), and here in English. It tells the next part of the story of the integration sites on the long arm of the law, I mean, on chromosome 9, and on chromosome 12 where the FAIM2 locus is found. Also go here for an excellent summary article by Rebekah Barnett.

The FAIM2 locus? What is that? What is a locus? Is it a locust without the t? But what would a locust be without t? He’s so cute!

On loci (locus = singular)
A locus is a specific site or place on a chromosome of a gene or a genetic marker. The nomenclature indicates the locus on the chromosome like this: 12q13.12, where the 12 refers to the chromosome number, the q refers to the long arm of the chromosome with regard to the centromere, the 13 refers to the location on the arm, and the 12 refers to a more precise location on the arm.
FAIM2 stands for Fas Apoptotic Inhibitory Molecule 2, and as its name states, it inhibits Fas (death receptor)-mediated cell death (self-destruct system for cells) and thus is very important for maintenance of homeostasis of cell populations. This particular molecule is relevant in the context of cancer/tumours.

Regulation of apoptosis is important for both tumor cell survival and death. FAIM2 is constitutively-expressed on neurons1920, and in addition to having protective effects for neurons21, it has indeed been shown to participate in regulating tumor initiation and progression.22 This is concerning because an integration event at or upstream of this site will likely alter the normal functioning of this gene. And since the normal functioning of this gene is to inhibit self-destruction of cells, for example, then maybe important cells will self-destruct, and maybe cancerous cells, won’t. See the problem?
FAIM2 over-expression is associated with neuroblastomas (adrenal glands).2324
A VAERS query yielded 5 neuroblastoma reports. Now, this doesn’t sound like many as per VAERS reports, but all of the neuroblastoma reports are for individuals older than 16. This is notable in that these types of cancers are rare to begin with, and are primarily reported in infants ages 1-2.
On single nucleotide polymorphisms (SNPs)
All of us human beings are unique. One of the reasons we are unique is because our individual genomes are not identical. Each of us have different Single Nucleotide Polymorphism (SNP) profiles, for example. SNPs are a naturally-occurring intra-species phenomena - as long as it occurs 1% of the time - and can affect disease state susceptibility or preponderance, such as is the case for Alzheimer’s susceptibility due to variations (polymorphisms) in the APOE gene, for example. What this means, is that at certain positions in our genomes, we have different letters, effectively dictating our genotypes and phenotypes. Interestingly, SNPs are conserved during evolution and have been linked to many human diseases.

Kevin found SNPs in the Pfizer vial-cell context, but not in the vial context alone. What this means is that these genetic variations are the by-product of multiple rounds of replication (instigated by the transfection) to produce mutated versions of specific segments of DNA. “We do not expect SNPs in the plasmid backbone unless the cells are replicating those sequences and making errors in the process.” The thing about copying DNA, is that even with excellent proofreading available in the context of DNA by DNA polymerase, ‘mistakes’ can occur.
This is extraordinary to me. Mistakes in assignment of a single nucleotide are occurring somewhere along the replication line effectively creating new SNPs. Wild.
What does all this jargon mean to real people?
Neither Kevin nor I know what the physiological implications are going to be in the human being. There’s no way to know because we’ve never done anything like this before and we have no data to compare it to from prior experiments or clinical trials.
Kevin wrote:
Small amounts of contamination can be amplified inside the cell making the current DNA regulation loop hole large enough to drive a truck through.
This doesn’t sound good to me, however.
Do you guys understand what he found though? This is exogenous, contaminating, integrating DNA, that is amplifiable in these cells. My mind goes to existing cancers in people injected with the modified mRNA products. What effect are these SNPs going to have on the cell? Are these integration events occuring on other chromosomes (other than Chr9 and Chr12)? Is there something unique about these loci that allowed the integration to ensue? Are these specific loci prone to mutation during replication or DNA repair?
This story just keeps unfolding, and it’s all because of the work of citizen scientists like Kevin and Ulrike (and many, many others). Imagine, just imagine, how we would have no knowledge of these results without them. And unlike the regulators and product pushers/designers, we are openly transparent with our findings.
For my part, I write these articles to help a broader audience understand the many aspects of wrong with regard to these products.
Final thoughts
Everything, is balance. If not for exogenous ‘imbalancers’ thrown at ‘normally-functioning’ systems, then disease and/or metabolic disorders, as we currently know them, would not be an issue. These exogenous imbalancers primarily include toxins in our environment due to mega-polluters - think changes in gene activity - epigenetics - as opposed to changes in DNA, but also include toxins that we inject, put on our skin, eat and drink. If one desired to promote health and longevity, and to prevent cancer from becoming too prolific (pun intended), one might aim to keep ROS to a minimum (avoid ‘processed/packaged/factory’ “food” and high fructose corn syrup), and avoid ‘other chemical agents’ or exogenous LNP-based RNA/DNA products.
Hetzer, Mertin (February 3, 2010). "The Nuclear Envelope". Cold Spring Harbor Perspectives in Biology. 2 (3): a000539. doi:10.1101/cshperspect.a000539
Young JL, Benoit JN, Dean DA. Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature. Gene Ther. 2003 Aug;10(17):1465-70. doi: 10.1038/sj.gt.3302021. PMID: 12900761; PMCID: PMC4150867
Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. (2010) 40:179–204. doi: 10.1016/j.molcel.2010.09.019
Mehta A, Haber JE. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb Perspect Biol. 2014 Aug 7;6(9):a016428. doi: 10.1101/cshperspect.a016428. PMID: 25104768; PMCID: PMC4142968.
Jiang H, Mei YF. SARS-CoV-2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro. Viruses. 2021 Oct 13;13(10):2056. doi: 10.3390/v13102056. Retraction in: Viruses. 2022 May 10;14(5): PMID: 34696485; PMCID: PMC8538446
Bradbury A, O'Donnell R, Drew Y, Curtin NJ, Sharma Saha S. Characterisation of Ovarian Cancer Cell Line NIH-OVCAR3 and Implications of Genomic, Transcriptomic, Proteomic and Functional DNA Damage Response Biomarkers for Therapeutic Targeting. Cancers (Basel). 2020 Jul 17;12(7):1939. doi: 10.3390/cancers12071939. PMID: 32709004; PMCID: PMC7409137
Beaufort CM, Helmijr JC, Piskorz AM, Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van IJcken WF, Heine AA, Smid M, Koudijs MJ, Brenton JD, Berns EM, Helleman J. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One. 2014 Sep 17;9(9):e103988. doi: 10.1371/journal.pone.0103988. Erratum in: PLoS One. 2015;10(3):e0122284. PMID: 25230021; PMCID: PMC4167545
Mitra AK, Davis DA, Tomar S, Roy L, Gurler H, Xie J, Lantvit DD, Cardenas H, Fang F, Liu Y, Loughran E, Yang J, Sharon Stack M, Emerson RE, Cowden Dahl KD, V Barbolina M, Nephew KP, Matei D, Burdette JE. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol Oncol. 2015 Aug;138(2):372-7. doi: 10.1016/j.ygyno.2015.05.040. Epub 2015 Jun 5. PMID: 26050922; PMCID: PMC4528621
https://www.medicalnewstoday.com/articles/316551#Strong-correlation-between-cancer-incidence-and-normal-cell-division
https://en.wikipedia.org/wiki/Cancer_epigenetics
https://en.wikipedia.org/wiki/Microhomology-mediated_end_joining
Liang L, Deng L, Chen Y, Li GC, Shao C, Tischfield JA (September 2005). "Modulation of DNA end joining by nuclear proteins". The Journal of Biological Chemistry. 280 (36): 31442–31449. doi:10.1074/jbc.M503776200
Würtele, H., Little, K. C. E., & Chartrand, P. (2003). Illegitimate DNA integration in mammalian cells. Gene Therapy, 10(21), 1791–1799. doi:10.1038/sj.gt.3302074
de Vries J, Wackernagel W. Integration of foreign DNA during natural transformation of Acinetobacter sp. by homology-facilitated illegitimate recombination. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2094-9. doi: 10.1073/pnas.042263399. PMID: 11854504; PMCID: PMC122324
Craigie R, Bushman FD. HIV DNA integration. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006890. doi: 10.1101/cshperspect.a006890. PMID: 22762018; PMCID: PMC3385939
Kaitao Zhao, Andrew Liu, Yuchen Xia, Insights into Hepatitis B Virus DNA Integration-55 Years after Virus Discovery, The Innovation, Volume 1, Issue 2, 2020, 100034, ISSN 2666-6758, https://doi.org/10.1016/j.xinn.2020.100034
Xu Y, Li Z. CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Comput Struct Biotechnol J. 2020 Sep 8;18:2401-2415. doi: 10.1016/j.csbj.2020.08.031. PMID: 33005303; PMCID: PMC7508700
Kumar TR, Larson M, Wang H, McDermott J, Bronshteyn I. Transgenic mouse technology: principles and methods. Methods Mol Biol. 2009;590:335-62. doi: 10.1007/978-1-60327-378-7_22. PMID: 19763515; PMCID: PMC4095860
Fernández M, Segura MF, Solé C, et al. Lifeguard/neuronal membrane protein 35 regulates Fas ligand-mediated apoptosis in neurons via microdomain recruitment. Journal of neurochemistry. 2007 Oct; 103(1): 190–203
Schweitzer B, Taylor V, Welcher AA, et al. Neural membrane protein 35 (NMP35) a novel member of a gene family which is highly expressed in the adult nervous system. Mol Cell Neurosci. 1998 Aug; 11(5-6): 260–73
Beier CP, Wischhusen J, Gleichmann M, et al. FasL (CD95L/APO-1L) resistance of neurons mediated by phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent expression of lifeguard/neuronal membrane protein 35. Journal of Neuroscience. 2005 Jul 20; 25(29): 6765–74
She K, Yang W, Li M, Xiong W, Zhou M. FAIM2 Promotes Non-Small Cell Lung Cancer Cell Growth and Bone Metastasis by Activating the Wnt/β-Catenin Pathway. Front Oncol. 2021 Sep 9;11:690142. doi: 10.3389/fonc.2021.690142. PMID: 34568020; PMCID: PMC8459617
Kang, H., Kim, J., Chang, H. et al. FAIM2, as a novel diagnostic maker and a potential therapeutic target for small-cell lung cancer and atypical carcinoid. Sci Rep 6, 34022 (2016). https://doi.org/10.1038/srep34022
Planells-Ferrer L, Urresti J, Soriano A, Reix S, Murphy DM, Ferreres JC, Borràs F, Gallego S, Stallings RL, Moubarak RS, Segura MF, Comella JX. MYCN repression of Lifeguard/FAIM2 enhances neuroblastoma aggressiveness. Cell Death Dis. 2014 Sep 4;5(9):e1401. doi: 10.1038/cddis.2014.356. PMID: 25188511; PMCID: PMC4540192






Dr. Rose, you create a sense of awe within me at your focus and intelligence in grasping concepts most cannot. You are for sure a gem, a veritable expert on this subject and more as well as a lovely woman whose morality shines in your desire to promote the Truth. Few will comment on this as it far transcends the scope of abilities that your mind possesses. You are indeed noble and true.
On June 12, 2022, after four Pfizer injections, my very healthy mom was suddenly diagnosed with stage-IV pancreatic cancer in her left inguinal groin lymph node, B-cell lymphoma, and melanoma. Her immune system had failed completely. The fast-growing tumors spread to her bones, breaking them from the inside. She lived, suffering, until December 13.
I was her full-time caregiver.
In 2023, day by day, using memories, photos, text conversations, medical records, my journal, and my mom’s journal, I chronicled the story of her disease on Facebook. I told about the progression of her illness, the failed medical response, her unimaginable pain, her experience, my experience,and how her spirit refused to be broken.
I am currently in the process of editing and rewriting, on Substack.
My mom represents millions of people who were deceived, intimidated or forced into receiving an injection. Her story is all of our story.
https://mamaearthdesignshop.substack.com?utm_source=navbar&utm_medium=web&r=368d5r