Aluminum-associated neuro-degenerative diseases and cancer
The essential role of zinc finger proteins
I decided to investigate potential effects of introducing aluminum via the IM route on zinc finger proteins (ZFPs) and more specifically, zinc finger domains (ZFDs). I was prompted to do so by a recent article written about by Maryanne Demasi recently that summarizes a cover-up (failure to to conduct proper tests with knowledge and intent to do so) by Merck with regard to its Gardasil vaccine. Apparently, it was contaminated with HPV DNA fragments and they knew and did nothing about it. You can read that here.
What does contamination of foreign genetic material have to do with aluminum and ZFPs/ZFDs, you ask? Well, aluminum binds DNA,12 and if that wasn’t interesting enough, aluminum can induce a whole lot of mischief in terms of ZFPs and disrupt their normal functioning which includes, well, everything, and can manifest as neuro-degenerative diseases and cancer.
What are ZFPs and ZFDs?
In a word, a ZFP is a protein that is effectively necessary to maintain a healthy being by binding DNA. They use zinc ions to stabilize their structure, particularly through interactions with cysteine and histidine residues. ZFPs are ubiquitous in biology found in ~3% of the genes of the human genome.3 There are many ZFP classes that are determined by their structure and ligand placement for coordination of zinc ions (Zn2+).
So, the ZFP is the entire protein molecule that includes ZFDs as part of its entity. The ZFD refers specifically to the structural motif (or domain) responsible for the binding functions within the ZFP. Each ZFD typically contains around 30 amino acids, with a zinc ion coordinated by cysteine and/or histidine residues. This coordination helps to stabilize the structure, forming a loop and an alpha-helix that create the 'finger' shape. This coordination stabilizes the protein structure by forming a tetrahedral complex around the zinc ion. The tandem nature of this coordination is the key to the binding event. Teamwork.
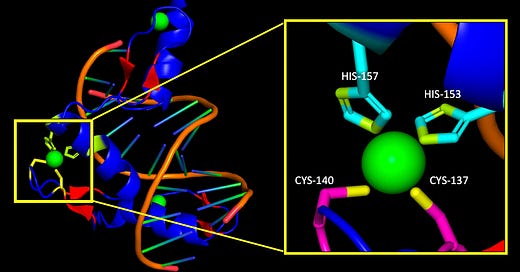
Figure 1 shows a zinc finger motif (repeating pattern)/domain (functional unit) (ZFD) that make up a ZFP. When you visualize a ZFP; think many ZFDs (See Figure 2). The green ball is the zinc ion, snug as a bug in the tetrahedral complex comprising two cysteines and two histidines to form the stabilized zinc finger: ready for binding.

In terms of ZFDs binding zinc ions to induce a DNA-binding event, think of it this way. Let’s assume we love baseball, and we were given a baseball glove and a baseball, both in a custom-built case. The glove and ball only fit into the case together when the baseball is inside the mitt of the glove. When the ball is in the mitt, they both fit in the case. This ‘fit’ is like our binding event to what have you, DNA, proteins, lipid substrates, that can occur with ZFPs. This is probably a terrible analogy.
Zinc likes the sulfers in cysteines and nitrogens in histidines, so this is where the bond takes place. The crystal structure of 1A1L is shown in Figure 2 (You can find this in the pdb database: ZIF268 zinc finger-DNA complex (GCAC site) here ). I blew up the tetrahedral complex formed between CYS-137, CYS-140, (yellow) HIS-153, HIS-157 (lime) and zinc (green) to give you an idea of what it looks like.

Isn’t is cool how nothing ever really touches?
Figure 3 depicts a ZFP with 3 ZFDs (blue) complexed to DNA (orange).4 N.B. It is referred to as a “cartoon” because this a simplified depiction meant to clarify the spatial relationship and interaction between Zif268 and DNA. The 3 zinc ions in Figure 3 are held by the amino acid residues, and this coordinated binding event induces a very particular shape. It is precisely this shape that allows subsequent binding to specific DNA motifs (GC boxes are commonly binding targets).5
Think of our ball-glove-box analogy. The baseball is the zinc ion, the ZFP is the glove, the places inside the glove that touch the ball are the ZFDs, and the box is the DNA (in this example). It is kind of inverted when you think about it since the box is on the outside, so maybe the box is the zinc and the ball is the DNA? I don’t know. I’m not into baseball. I prefer soccer, or football, as some like to call it. Anyway, you get the idea of the necessity for coordinated binding for conformation and subsequent more binding, I hope.

Because of the role of ZFPs as transcription initiation complexes to bind DNA-specific regions, they are crucial in terms of cell development, differentiation, metabolism, and autophagy. And if you’re starting to think about its role in cancer progression, you’re thinking right! From DNA recognition, RNA packaging, transcriptional activation, regulation of apoptosis, protein folding and assembly, and lipid binding, their roles are countless and incredibly important to health and normal functioning. It has been shown that messing with these proteins leads to a variety of human diseases, ranging from neuro-developmental disorders to cancers.6
But I’m more interested in why we see physiological effects like neuro-degeneration and cancer. So basically, I am more interested in the link to neuro-degeneration and cancer in terms of what would happen in a lot of aluminum came into the ZFP milieu (the human body).
Questions:
Would the aluminum ions be able to interact with the ZFDs?
If they did interact with the ZFDs, what effect on the protein’s structure would it have, and would it potentially mess up its ability to bind DNA?
Would they be able to defer binding of zinc ions to destroy original functionality?
Let’s dig into some answers.
Yes.
In spite of the fact that there's no natural biological system designed to incorporate aluminum into such a critical role as played out by ZFPs, aluminum can interact with/bind to ZPDs. The good news is that it is not a great affinity bond because aluminum has a smaller ionic radius than zinc. Basically all that means is that if there’s both zinc and aluminum around, zinc will out-bind aluminum. Binding aluminum in the ZFP context would be like trying to grasp a grain of sand with your hand: you could have it in your hand but if you move some way, the grain of sand would drop out. Where zinc forms stable (often reversible bonds with sulfur (from cysteine) and nitrogen (from histidine)) crucial for maintaining the protein's structure and function, aluminum tends to form less stable bonds with sulfur, but this can lead to protein instability or mis-folding. By the way, aluminum also has a higher affinity for oxygen, which could lead to unwanted interactions with other parts of the protein or cellular components.
Functionally, the specific geometry and electronic properties of zinc are crucial for the DNA recognition and binding functions of ZFPs. Substituting aluminum could lead to loss of function or altered specificity in DNA interactions. So it’s safe to say that if a buttload of aluminum was introduced to a person, considering that aluminum can occupy the zinc binding, and add to that that some people are zinc deficient, that this could theoretically lead to functional problems with respect to ZFPs. This in turn, could lead to health problems - neuro-degeneration and cancers.
If aluminum ions were to bind, they might not only fail to mimic zinc's role but could also introduce toxicity, as aluminum is known for its potential neurotoxic effects and is not regulated by the body in the same way zinc is.
Aluminum prefers a higher coordination number, typically forming octahedral complexes rather than the tetrahedral ones favored by zinc and this could lead to protein mis-folding. Not good. We need those proteins folded properly to effect their functions. For example, the protein might not fold correctly since the binding sites are designed for a different metal ion geometry, and thus, they may not bind some DNA that they need to in order to maintain a healthy state. Aluminum interaction/binding could also lead to other types of structural distortion and/or destabilization of the protein, and increase its susceptibility to degradation. It could also lead to protein aggregation, similar to what is observed in some metal-induced proteinopathies. Where have I heard that before?
Even more concerning, it could lead to changes in transcriptional regulation: any change in this realm could lead to dysregulation of gene expression, potentially causing cellular or systemic dysfunction. If said proteins are involved in DNA repair, altered function could increase mutation rates or genomic instability. Oh dear.
Yes.
I am going to leave this as a teaser for now because I need to do more reading, but please think about this and do some literature scouring on this subject matter. Nicole Shanahan has recently put out a call for people to put together a review of papers that link gut to autism to vaccines, and I am almost certain that the answer lies in aluminum effects on ZFPs.
*I am also wondering whether the effects of consistent spraying of our skies (ie: geoengineering/stratospheric aerosol injection) with light reflecting aluminum particles (aluminum particles are used to form a reflective layer in the stratosphere which aim to reflect a small percentage of the sun's light back into space to cool the planet - roll eyes here) is not having a far worse effect on us than we may have thought in terms of all these cancers we’re seeing. Don’t forget, there's no natural biological system designed to incorporate aluminum.
Think about that.
H. J. Lozano, N. Busto, M. Lari, J. M. Leal and B. García, New J. Chem., 2018, 42, 8137 DOI: 10.1039/C8NJ01779D
Karlik SJ, Eichhorn GL, Lewis PN, Crapper DR. Interaction of aluminum species with deoxyribonucleic acid. Biochemistry. 1980 Dec 23;19(26):5991-8. doi: 10.1021/bi00567a008. PMID: 7470444
Klug A (2010). "The discovery of zinc fingers and their applications in gene regulation and genome manipulation". Annual Review of Biochemistry. 79: 213–31. doi:10.1146/annurev-biochem-010909-095056
Kendrew JC et al. (1958) A three-dimensional model of the myoglobin molecule obtained by X-ray analysis. Nature 181:662–6
Lundin M, Nehlin JO, Ronne H (March 1994). "Importance of a flanking AT-rich region in target site recognition by the GC box-binding zinc finger protein MIG1". Molecular and Cellular Biology. 14 (3): 1979–85. doi:10.1128/MCB.14.3.1979
Kamaliyan Z and Clarke TL (2024) Zinc finger proteins: guardians of genome stability. Front. Cell Dev. Biol. 12:1448789. doi: 10.3389/fcell.2024.1448789




You plant a teaser, and so will I. Years back, as in wayback,
https://web.archive.org/web/20080704102226/http://www.drcarley.com./
I read a Dr. Rebecca Carley who was adamant that autism was due to the MMR, not mercury or other. In the article, search for SSPE, which is SUB ACUTE SCLEROSING PAN ENCEPHALITIS.
Dr. C quotes "Harrison's Principles of Internal Medicine" (I think it is an early 1970's edition):
"In the aforementioned 6th edition of Harrison's Principles of Internal Medicine on p. 1791, under the heading "Multiple Sclerosis and other DEMYELINATING Diseases", it states the following: "A large and important group of neurologic disorders are termed the demyelinating diseases because they share the common pathologic feature of foci of degeneration, involving the myelin sheath of nerves. ... acute disseminated encephalomyelitis (including post infectious and POSTVACCINAL ENCEPHALOMYELITIS)...(P. 1798) - "the association of the neurologic disorder with vaccination or inoculation usually leaves the diagnosis in little doubt, and the characteristic combination of encephalitic and myelitic features will help to distinguish the condition from meningitis, viral encephalitis, and poliomyelitis". This self evident fact has been taken out of subsequent editions of Harrison's Principles of Internal Medicine"
According to Dr. Carley, SSPE is now called Autism.
"Nicole Shanahan has recently put out a call for people to put together a review of papers that link gut to autism to vaccines, and I am almost certain that the answer lies in aluminum effects"
Have you ever read the research of Christopher Exley? He's researched aluminum for over 40 years and has studied the health effects of AL in the human body (including via vaccines, food, etc.). He wrote a 2020 book called "Imagine You Are An Aluminum Atom: Discussions With Mr. Aluminum"
Just this morning, I read his new Substack post @ "Dr Christopher Exley from Dr’s Newsletter" where he wrote "fluoride increases the toxicity of aluminium" ... "increases the absorption of aluminium across the gastrointestinal tract" ... "an alternative explanation as to why fluoride exposure in potable water is contributing to neurodevelopmental conditions in infants and adolescents and neurodegenerative disease in adults." https://drchristopherexley.substack.com/p/fluoride-chemistry-and-human-health