A question and answer document on the subject of VAERS as a pharmacovigilance tool.
Feel free to print and use.
Dr. Jessica Rose - A short Q and A - August 9, 2022
1. "Are the COVID vaccines causing injuries, what kinds, and how do we know?"
Yes. Any human physiological system that you can name, able to be affected in an injurious way, is being affected in an injurious way in a percentage of individuals. We know because VAERS – a pharmacovigilance tool – currently has an extraordinarily high number and range of adverse event reports in the context of the COVID-19 injectable products. We also know because the Yellowcard and EUDRA adverse event (AE) data collection systems are reporting the same things.[1]
2. An introduction to VAERS: what is it, who runs it, what is its history?
VAERS[2] is a pharmacovigilance tool designed to detect safety signals (not detected in pre-market testing) emitted in people following administration of biological or pharmaceutical products. It was originally put into place in 1990 as a solution to non-liability of pharmaceutical companies as per the National Childhood Vaccine Injury Act of 1986 (NCVIA)[3]. In spite of the fact that the NCVIA requires health care providers and vaccine manufacturers to report to the U.S. Department of Health & Human Services (DHHS)[4] specific AEs following the administration of vaccines outlined in the Act, under-reporting is a known imperfection of the VAERS system.[5],[6] VAERS is maintained and monitored by the Centers for Disease Control (CDC)[7] and the Food and Drug Administration (FDA)[8].
3. In fairness to the "other side", what is the distinction between reported injury and confirmed injury?
A reported injury that gets into the front-end downloadable database IS a confirmed injury.[9]
4. What is the reality of submitting an adverse event report to the VAERS system (the difficulty and the time investment involved on front end and in follow up)?
It takes 30 minutes – at least – to file a VAERS report online. It requires a multi-electronic page submission of detailed information and each electronic page is time-restricted. This means that if one does not complete the electronic form fast enough, one must begin the submission process again. The vetting process is long and dealt with by a handful of contracted employees.[10] There is a massive backlog of data in VAERS.[11]
5. What percentage of actual incidents is reflected by the number of reports - and how are we making that guess?
The under-reporting factor represents the percentage of individuals who actually experienced an adverse event in contrast with how many individuals reported an event. Studies have shown that the percentage of incidents reported can be quite low (1–10%). My own study showed an under-reporting factor of 31, which means that each stand-alone adverse event count must be multiplied by a factor of 31.[12],[13],[14] Steve Kirsch also estimated the under-reporting factor to be 41 based on Anaphylaxis data.1
6. What are the top injuries reported with percentages?
Cardiovascular and neurological injuries remain highest with regard to grouped adverse event reporting frequency.[15],[16]
7. How does this injury rate compare to all other vaccines recorded by VAERS?
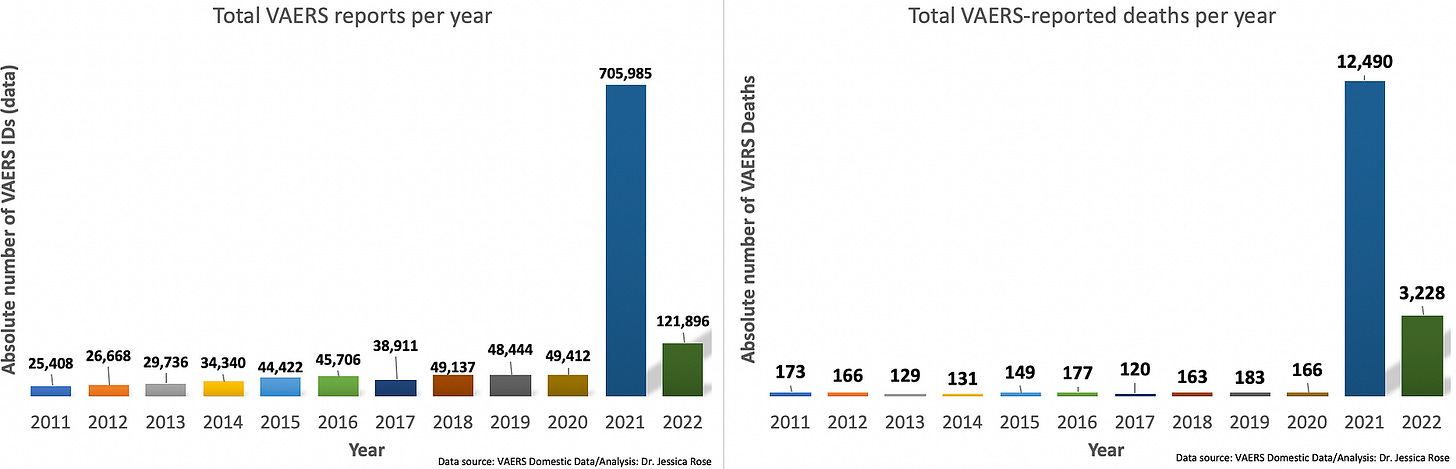
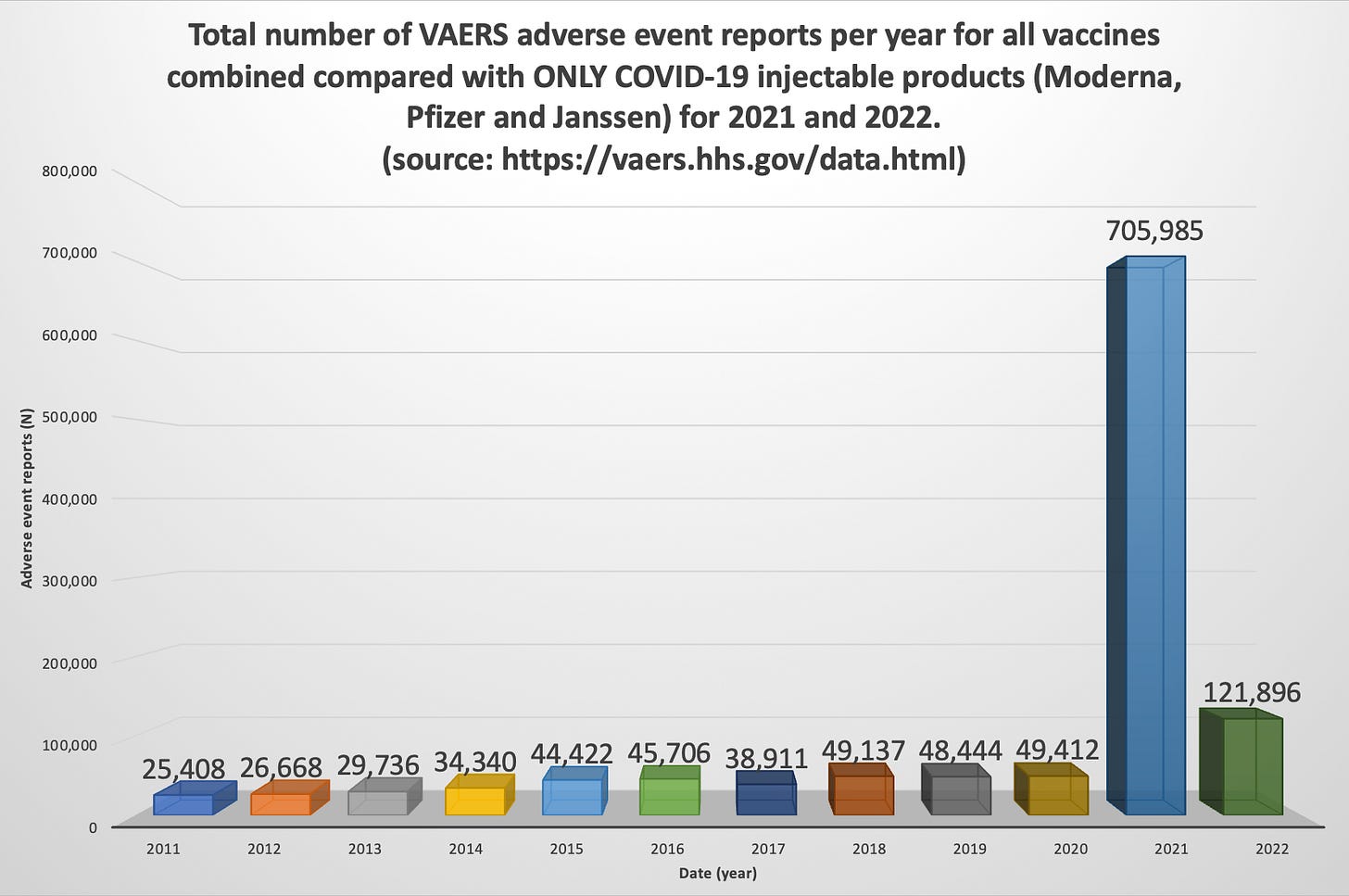
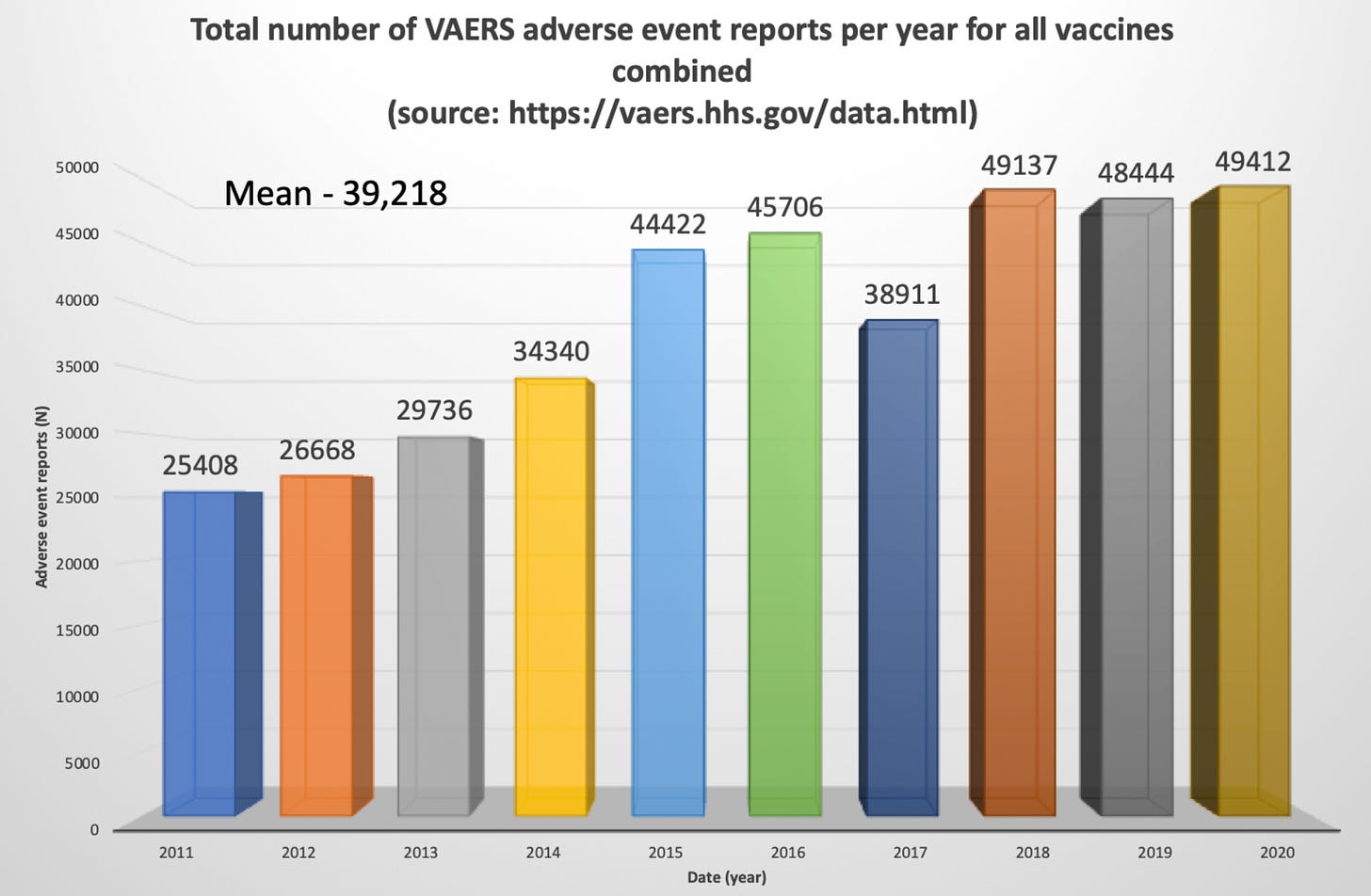
The injury rates do not compare in any way, shape or form to all other vaccines combined for the past 30 years. For the past 30 years, the VAERS data has housed approximately 39,000 adverse event reports per year for all vaccines combined. Of these, approximately 155 have been deaths. In 2021, there were 705,985 reports and 12,490 deaths in the context of the Moderna[17], Pfizer/BioNTech[18] and Janssen[19] products, in the VAERS Domestic data set alone, not including all other products on the vaccine list.
8. Request: Please include the narrative on that amazing CDC ad looking for analysts to cover the 770,000 incidents related to COVID alone.
In reference to a previous article that I wrote on the subject of the VAERS support contract going up for rebid, it was documented that the new VAERS contractor would provide VAERS follow-up activities and enhanced surveillance and quality control for VAERS data.
In the proposal, it is written:
“The contractor shall implement a staffing and operations plan focusing only on vaccine adverse event (VAE) reports after COVID-19 vaccines to help process an estimated 770,000 digital reports per year. Periods of heavier and lighter reporting volume are anticipated throughout the year. Contractor is responsible for maintaining active monitoring and adjusting of the number of reports processed on an annual basis.”
This means one thing: The CDC are well aware of the number of adverse event reports being filed in VAERS in the context of these COVID-19 shots, and how this contrasts starkly to historical report counts.
The CDC know: they are recruiting new employees to deal with the large number of individuals who they anticipate to be submitting adverse event reports in the context of the COVID-19 shots, and they are compensating in this regard without public acknowledgement.
This is a bar graph showing the total number of adverse events reported to VAERS (per year) for all vaccines combined going back 10 years. Notice the mean is 39,218 reports and the peak number of events occurs in 2020 at 49,412.
Thank to Ken McCarthy for instigating/inspiring this report.
[1] https://vaers.hhs.gov/data/datasets.html
[2] https://vaers.hhs.gov
[3] https://www.congress.gov/bill/99th-congress/house-bill/5546
[4] https://www.hhs.gov/
[5] Lazarus, Ross et al. Grant Final Report. Grant ID: R18 HS 017045. Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS). Submitted to The Agency for Healthcare Research and Quality (AHRQ).
[6] Rose, J. 2021, Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System? Science, Public Health Policy & the Law Volume 3:100–129.
[7] https://www.cdc.gov/
[8] https://www.fda.gov/
[9] https://www.cdc.gov/vaccinesafety/pdf/VAERS-v2-SOP.pdf
[10] https://www.cdc.gov/vaccinesafety/pdf/VAERS-v2-SOP.pdf
[11] Rose, J. 2021, Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System? Science, Public Health Policy & the Law Volume 3:100–129.
[12] Lazarus, Ross et al. Grant Final Report. Grant ID: R18 HS 017045. Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS). Submitted to The Agency for Healthcare Research and Quality (AHRQ).
[13] Crawford M. 2021. How Underreported Are Post-Vaccination Serious Injuries and Deaths in VAERS? The Chloroquine Wars Part LI. https://roundingtheearth.substack.com/p/how-underreported-are-post-vaccination
[14] Rose, J. 2021, Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System? Science, Public Health Policy & the Law Volume 3:100–129.
[15] Rose, J. 2021, Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System? Science, Public Health Policy & the Law Volume 3:100–129.
[16] Rose, J. 2021. A report on US Vaccine Adverse Events Reporting System (VAERS) of the COVID-19 messenger ribonucleic acid (mRNA) biologicals. Science, Public Health Policy & the Law. 2:59-80.
[17] https://www.modernatx.com/en-US
[18] https://www.pfizer.com/products/product-detail/comirnaty
[19] https://www.janssenmd.com/janssen-covid19-vaccine
http://www.skirsch.com/covid/Deaths.pdf


Nice job. URF=41 which is based on anaphylaxis studies. Your number is based on gamed clinical trials.
It continues to boggle the mind when you look at prior vaccines and the numbers of adverse events needed to pull vaccines and compare it to this covid thing. From CDC: "On July 16, 1999, CDC recommended that health-care providers suspend use of the licensed rhesus-human rotavirus reassortant-tetravalent vaccine (RRV-TV) (RotaShield®, Wyeth Laboratories, Inc., Marietta, Pennsylvania) in response to 15 cases of intussusception". From iastate.edu, "The
catastrophic vaccine failure of a formalin-inactivated (FI) RSV vaccine from 1966. During one
study, 80% of infants given the vaccine developed severe bronchiolitis or pneumonia after wildtype infection, compared to only 5% for the placebo group. Ultimately two of the vaccinated
infants died (Kim et al., 1969)." Now we have ten's of thousands of severe reactions and deaths and all we hear is let's inject another group and another with another and another. Insanity reigns supreme.