Spike Protein Induced Cardiotoxicity: A Potential Tsunami of Cardiac Issues Over the Next 20+ Years
How the Spike Protein’s mimicking of radiation damage induces cardiotoxicity
I dedicate this post to Peter McCullough, for his courage and unstoppable search for the truth.
Building on my previous work and the work of others, readers will recall how I have compared the damage the Spike Protein of SARS-CoV-2 causes to that of radiation. The exact same pathology that is induced by radiation is mimicked by the Spike Protein. This has both immediate and long-term consequences. It may be that, as with radiation induced cardiotoxicity, the damage may be irreversible.
If we examine the mechanisms of radiation induced cardiotoxicity, we observe the parallels to what I will now call Spike Protein Cardiotoxicity.
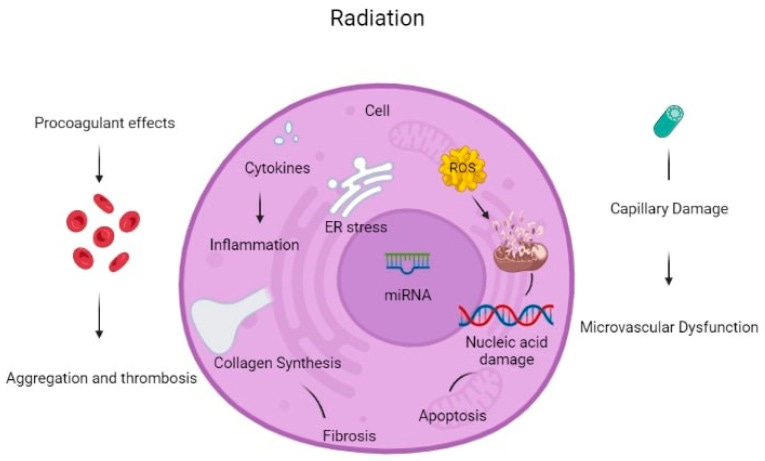
There are many reported mechanisms of cardiotoxicity caused by radiation therapy exposure in cancer patients. Cardiac cell dysfunction due to oxidative stress from radiation exposure is an important mechanism of cardiac toxicity. Mitochondrial dysfunction due to radiation can break the respiratory chain of metabolism, causing an increase in the production of Reactive Oxygen Species (ROS). Antioxidant capacity cannot override this increase, leading to mitochondrial instability and dysfunction. ROS can cause macromolecule (lipid, protein, amino acid, etc.) damage. Apart from increased ROS formation, direct DNA damage from radiation leads to DNA strand breaks, causing genome instability. Impaired DNA correction mechanisms are not working properly under radiation exposure, becoming unable to correct the apparent DNA damage, leading to the activation of apoptotic mechanisms [8].
Radiation leads to macromolecule damage causing cardiac cell damage. Protein oxidation under increased ROS production cause amino acid chain breaks, misfolding and cross-linking, and dysfunction of degrading protease proteins. Many cytocellular metabolic mechanisms are affected because proteins function in many different cellular components, e.g., as receptors, enzymes, and transporters. Furthermore, ROS induces lipid peroxidation causing polyunsaturated fatty acid (PUFA) to form fatty acid radicals. Many antioxidative and other cellular membrane mechanisms can be deactivated by this transformation. All the above radiation-induced mechanisms cause molecular signaling pathway damage and cause cardiac toxicity [8].
Oxidative stress increases TGF-β1 leading to radiation-induced vascular injury and endothelial dysfunction. Furthermore, NF-kB upstream tumor necrosis factor (TNF) and interleukin (IL-1) production create a pro-inflammatory environment for cardiac cell inflammation and dysfunction [8]. All the aforementioned mechanisms can activate cellular apoptosis.
Furthermore, even 8 Gy of radiation dosage can stimulate the release of calcium from the endoplasmic reticulum to the cytoplasm leading to an increase in cytoplasmic calcium concentration. The above mechanism can increase mitochondrial ROS production and activate p53 promoting the accumulation of pro-inflammatory and prothrombotic molecules and increasing the probability of thrombosis, myocardial ischemia, and inflammation. Radiotherapy can be an alternative factor of atherosclerosis through capillary rupture and microvascular damage [9].
Non-coding RNAs (ncRNAs) are genetic, epigenetic, and translational regulators. NcRNAs mainly compose of long non-coding RNAs (lnc-RNAs), microRNAs (miRNAs), and circular RNAs (circRNAs). NcRNAs play an important role in apoptotic mechanisms (Fas pathway and caspase pathway cascade), mitochondrial damage (detoxification mechanisms disruption), oxidative stress (increase ROS production), autophagy, calcium homeostasis, and fibrosis. Radiation-induced miR-34a promotes gene aging and decreases the length and activity of nucleic acid telomeres. All the above induce cardiomyocyte aging and disruption. Some miRNAs can be used as a biomarkers for the early detection of cardiac cell toxicity after radiation therapy and early detection of CVD but they also can be used as potential therapeutic targets to reverse these toxic effects [10].
In addition, the following mechanisms cause CVD after radiation therapy. Interstitial fibrosis cause subsequent diastolic dysfunction left ventricular dysfunction, or left ventricular remodeling, and fibrosis of the conduction system may provoke arrhythmias or conduction defects. Pericardial fibrosis is important for many pericardial syndromes [11].
Furthermore, radiation exposure cause endothelial changes, which result in the reduced capillary-to-myocyte ratio, damage to epicardial vessels, and disrupt the balance between antithrombotic and prothrombotic, as well as anti-inflammatory and pro-inflammatory mechanisms. Induced monocyte and macrophage accumulation to the atherosclerotic plaque as well as enhancement of the action of classic cardiovascular risk factors, increase and accelerate the process of atherosclerosis in cancer patients. Studies in murine models after irradiation demonstrate structural changes in atherosclerotic plaques leading to more intraplaque hemorrhages and plaque instability that is vulnerable to thrombosis [12].
Furthermore, pathophysiological changes of radiation treatment exposure and induced cardiac toxicities have been studied in animal models, and information about fractionation of dosage and radiation protection treatments have been tested [13].
Deposition of extracellular matrix and fibrosis deteriorates the elastic structure of vessels, resulting in arterial rigidity. Many inflammatory molecules and cytokines play a detrimental role in this process, such as tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, platelet-derived growth factor (PDGF), monocyte chemotactic factor (MCF), and transforming growth factor (TGF)-b. Endothelial damage from radiotherapy disrupts endothelial-derived production of vasodilator factors, such as nitric oxide (NO). The consequent vasoconstriction and the activation of the prothrombotic environment can lead to hypoperfusion and microvascular damage [14]. Histological studies report two main complications on arteries: intimal disruption and hyperplasia and luminal stenosis or occlusions [15,16].
Another complication of radiation therapy consists of autonomic dysfunction. It may induce abnormal heart rate recovery time and increased resting heart rate [12]. Clinical radiotracer studies suggest that myocardial alterations in metabolic uptake are present in irradiated heart tissues, which are not linked to vascular territories of coronary arteries. Metabolic shifts may occur due to switching from fatty acid oxidation to glycolysis, eventually leading to increased glucose uptake, mitochondrial damage and radiation-induced heart disease (RIHD), and ischemic heart failure [17]. Zebrafish models are used for the characterization of the pathophysiology of acute, subacute, and delayed RIHD and for the identification of potential cellular and molecular therapeutic targets [18].
I apologize for the above extensive quote, however, all of it is critically relevant. From DNA to Endothelial damage. From deposition of extracellular matrix to fibrosis and autonomic dysfunction we see the complete panoply of Spike Protein pathology.
Why does this disturb me so much, and why should it disturb us all? Because the effects of radiation induced cardiotoxicity often does not become apparent for years, perhaps decades after the event.
The Spike Protein, I believe, is orders of magnitude more powerful than the amount of radiation given in cancer treatment. This timeline is almost certainly immensely condensed in the context of the Spike Protein.
Radiotherapy can cause radiation-induced heart disease. There is a different timeline from radiation treatment exposure until the cardiotoxic effects appear. Additionally, every distinct cardiac toxicity (CAD, VHD, cardiomyopathies, pericardial syndromes, conduction abnormalities) needs a different time from exposure to appear.
Cardiomyopathy incidence increases after radiation exposure. Non-ischemic cardiomyopathies, due to the direct effects of radiation and interstitial fibrosis, were observed in cancer patients. A shorter timelapse of around 3–4 years median time from radiation is needed for these clinical toxicities to appear. Most cases are clinically with heart failure symptoms.
Moreover, the damage is DOSE DEPENDENT and the YOUNGER THE SUBJECT IS AT TIME OF INITIAL EXPOSURE, THE MORE SERIOUS THE CONSEQUENCES.
There are many risk factors deteriorating cardiotoxic effects of radiotherapy, such as cumulative radiation dose, irradiated heart volume, young age at the time of radiation exposure (especially doses >30–50 Gy), fractional dosage (>2 Gy), heart volume irradiated, young patient age at the time of radiation exposure, the time elapsed since exposure, concomitant use of other chemotherapeutic agents and classical cardiovascular risk factors presentation [11]. Multiple factors, including cellular composition, differentiation, cell replication capacity, and cellular radiation sensitivity, determine the extent of possible toxicities [26]. The adult heart is considered unable to regenerate after fully developed and thus has been viewed as a radioresistant organ.
Radiation Treatment Mechanisms of Cardiotoxicity: A Systematic Review
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10094086/
I also apologize if this comes across as fear mongering. I assure you it is not. We must face this and cannot sweep it under the rug or brush it aside with abandon. If this hypothesis is true, we must immediately begin screenings and implement treatment protocols. Again, my fear is that, currently, much of the damage from radiation induced cardiotoxicity is irreversible.
I will continue to work towards complete understanding and successful treatment.