Once upon a time there was a recent FOIA request made that led nowhere. No! This isn’t the story of the PRR! This is another story of a nowhere lead. It seems to be par for the course these days, doesn’t it? Thanks to all the hard-working people requesting this information via FOIA, and also to all of the people trying to find the requested information in the piles of well, nothing. I guess it’s not that hard to sift through nothing.
Background
There are two documents that mention assessment of causality using adverse event data: “Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, 2nd ed., 2019 update” (World Health Organization (WHO)) and the “Standard Operating Procedures" (SOP) document updated Jan 29, 2021” (Centers for Disease Control (CDC)).
Acronyms to know:
FOIA: Freedom of Information Request
AE: Adverse Event
AESI: Adverse Event of Special Interest
AEFI: Adverse Event Following Immunization
SOP: Standard Operating Procedures
CDC: Centers for Disease Control
WHO: World Health Organization
CDC - The latter has a description of causality assessment protocol that goes like this:
2.0 Overview of VAERS Surveillance Activities
The specific tasks and frequency of these tasks for surveillance will be adjusted to meet public health needs, with consideration of staff time and resources. For example, in the event of a significant increase in the number of adverse events (AEs) reported to VAERS that warrant clinical review, additional ISO staff will be assigned to perform reviews. An algorithm of the process to monitor vaccine AEs is shown in Appendix 4.1.CDC will perform clinical reviews for AESIs listed in Table 1. Results from automated data assessment will identify additional conditions potentially warranting further clinical review.
Table 1 includes the following AESIs: Acute myocardial infarction (AMI), Anaphylaxis, Appendicitis, Bell’s Palsy, Coagulopathy, COVID-19, Death, Guillain–Barré syndrome, Kawasaki’s disease, Multisystem Inflammatory Syndrome in Children (MIS-C), Multisystem Inflammatory Syndrome in Adults (MIS-A), Myopericarditis, Narcolepsy/Cataplexy, Vaccination during pregnancy, Seizure, Stroke, Transverse myelitis. Pretty short list, but alright.
Thus, it is written in this document updated on January 29, 2021, that the AEs listed above would be clinically-reviewed by the CDC to identify conditions warranting further review, ie: causality assessment.
For my splendid readers, the VAERS reports without considering an Under Reporting Factor (URF) (as of July 1, 2022) are as follows. These counts are bound to be drastically underestimated (due to many factors such as multiple MedDRA codes for the same AE), and do not forget the URF. I have added the AE counts as they would be with an URF of 31 in brackets (URF of 10 for death) as well. Check out COVID-19.
Acute myocardial infarction (AMI): 6,090 (188,790)
Anaphylaxis: 9,526 (295,306)
Appendicitis: 1,560 (48,360)
Bell’s Palsy: 6,582 (204,042)
Coagulopathy: 516 (15,996)
COVID-19: 608,213 (18,854,603) - This is ironic, Alanis. Why don’t you sing about this? By the way, this is #1 reported AE in VAERS currently.
Death: 32,671 (w/URF 10 = 320,671)
Guillain–Barré syndrome: 2,319 (71,889)
Kawasaki’s disease: 46 (1,426)
Multisystem Inflammatory Syndrome in Children (MIS-C): 90 (2,790)
Multisystem Inflammatory Syndrome in Adults (MIS-A): 22 (682)
Myopericarditis: 37,803 (1,171,893)
Narcolepsy/Cataplexy: 178 (5,518)
Vaccination during pregnancy: 9,674 (299,894)
Seizure: 14,303 (443,393)
Stroke: 16,520 (512,120)
Transverse myelitis: 325 (10,075)
Appendix 4.1 is a schematic of the algorithm meant to be implemented to do these assessment and reviews as shown in Figure 1. You’ll note in red that the assessment of listed AESIs is part and parcel as part of the steps in the algorithm. You’ll also notice that the PRR signal is meant to be measured here. We all know by now that they also are not assessing the PRR thanks to that other FOIA request.

WHO - The former has a description of causality assessment protocol that is quite thorough. If you click on the link above, you’ll see that it takes you to a book! A book written about how to assess causality from adverse events. It is quite thorough! They define an AEFI as the following:
Adverse event following immunization (AEFI): This is defined as any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the use of the vaccine. The adverse event may be any unfavourable or unintended sign, an abnormal laboratory finding, a symptom or a disease.
They describe therein how to make these assessments both at the individual and the population levels. This is done using several of the Bradford Hill Criteria such as Temporality, Dose response and biological plausibility as shown in Figure 2.

Causality assessment usually will not prove or disprove an association between an event and the immunization. It is meant to assist in determining the level of certainty of such an association. A definite causal association or absence of association often cannot be established for an individual event.
Yes. It is meant to assess whether or not a biological product is doing harm to the public or subpopulations of the public. That’s what pharmacovigilance tools like VAERS are for: they represent the epidemiological data set from where the AEFIs are gathered.

As of July 1, 2022, in the context of AEs associated with COVID-19 injectable products reports filed in VAERS without considering an URF (URF 10 or 31), there are 32,671 (320,671 - URF 10) deaths, 30,933 (958,923) life threatening reports, 287,731 (8,919,661) hospitalizations, 51,438 (1,594,578) disabilities, 472 (14,632) birth defects reported, 312,839 (9,698,009) severe AEs reported, 63,795 (1,977,645) reports of hepatological AEs, 46 (460 - URF 10) Creutzfeldt-Jacob disease (CJD) reports, 4,554 (141,174) reports of spontaneous abortion, 25,910 (803,210) reports of diabetes, 26,402 (818,462) reports of cancer, 37,803 (1,171,893) reports of myocarditis and 79,936 (2,478,016) reports of thrombotic AEs, just to name a few clustered cases in VAERS. By the way, that CJD count is above the background reporting rate for the year. It is very concerning.
I think we have a Case.
The following is a screenshot of the WHO Causality assessment algorithm schematic on page 36 of Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, 2nd ed., 2019 update, that was sent with the FOIA request.
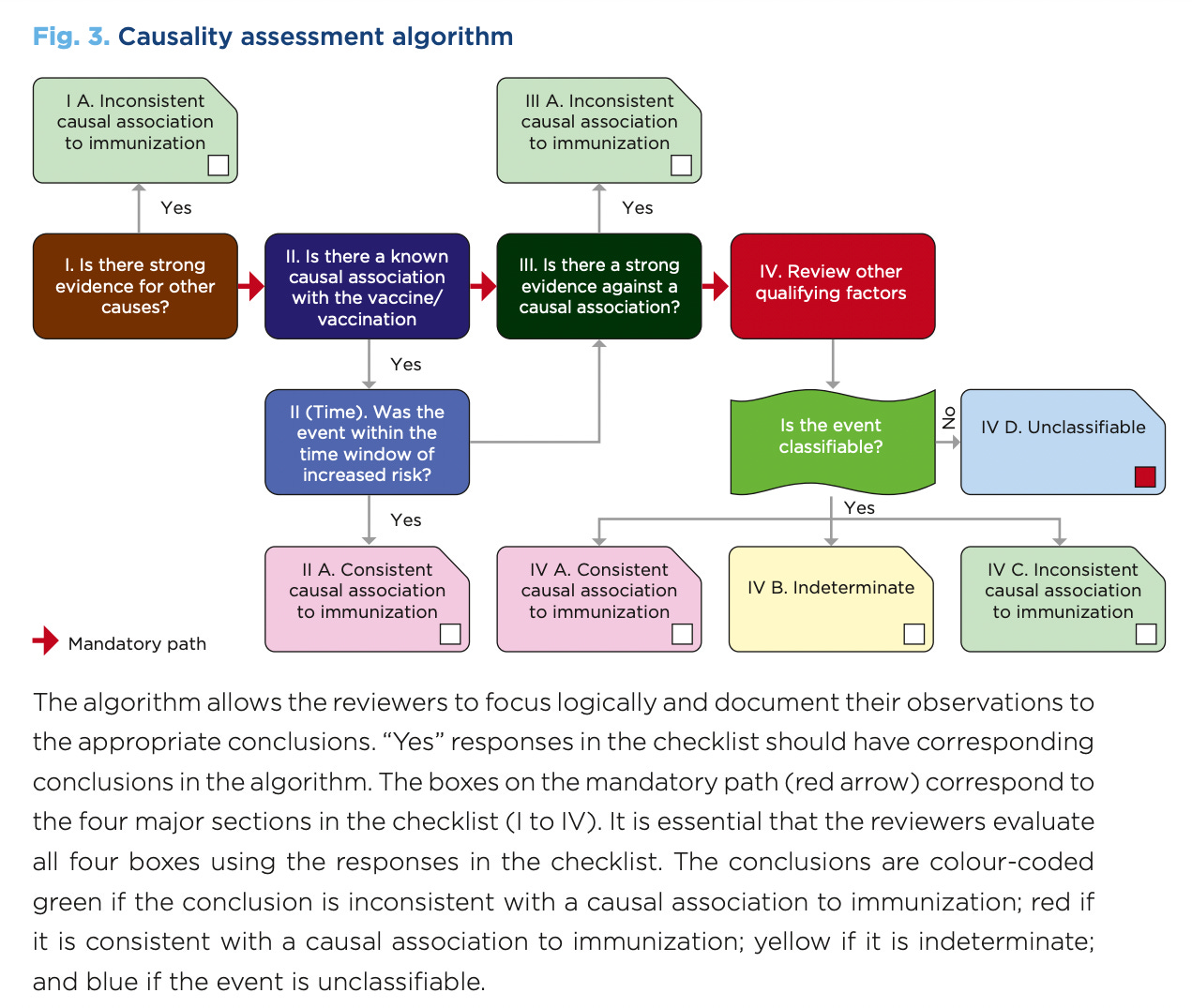
Just so everyone knows, they have used (and do use) VAERS data as the source data for this causality assessment protocol, in the past. In the example in Figure 5, they found no causal link between MMR shots and death reports in VAERS for kids. Of course they didn’t. That would halt the program.

So the FOIA request itself is valid, in my opinion. Here is the FOIA request letter.
Here is the expedient reply from the Department of Health and Human Services.
I can imagine that there might be some crafty word play at play here to side-step disclosing the information requested in the request. Or perhaps the request should have been more specific? Or less specific? Or perhaps the inclusion of the algorithm itself was a mistake? Or perhaps specifically requesting this causality assessment using VAERS data was the mistake? It is noteworthy, however, that the word ‘or’ was used to imply that the search should be made pertaining to both documents separately, but perhaps since the causality algorithm itself is associated with the WHO and not the SOP, it might understandable that they came up with nothing… ?
But still.
WHO’s doing the causality assessment? (Get it?)
Notice, that they did point the FOIA requester to the VAERS website to remind said requester that VAERS isn’t actually used for anything useful and remains the duck decoy for the fact that vaccine companies remain liable-less.






A comment from a great colleague: "The FOIA was too specific and gave them a get out of jail free card. It needs to be resubmitted with something like "Please disclose in detail CDCs methodology for determining causality, including any protocols or standard operating procedures. Please disclose the results from all analyses conducted under those methods, protocols or procedures." Learning all the time. I love Substack.
I wonder what % of the vax victims don't receive a diagnosis at all & are completely missed by the datasets, eg if you get sent home with Xanax obviously you won't generate an ICD.
Definitely a *lot* of stories from vax victims where they were spurned by the docs.