This took all day, and it is worth mentioning...
More oopsies in the world of court-ordered released data
Update: Question: Are CRFs only reported for those who suffered an adverse event such as death? One of the comments below provides insight into this question. The commenter wrote that only certain participants are required to have a CRF created for them according to ‘guidelines’.
CRFs are only required for subjects who experience a serious adverse event, death, an adverse event that is predefined in the study protocol as being of interest, and withdrawal due to an adverse event.
This appears to be confirmed in this document where on page 99 it is written:
In addition, the reports are required to include the case report forms (CRFs) for each patient who died during a clinical study or who did not complete the study because of an adverse event (unless this requirement is waived). The applicant shall submit these reports (1) 4 months after the initial submission…
Alright then. Let’s assume for a moment that things were done correctly here and that this reputable and benevolent mega-company did everything by the book. I can tell you with certainly, that nothing in this data is by any book. It is beyond contrived and don’t forget, they wanted to hide this data for 75 years from the public.
Then again, Houston, we have a problem.
If you scroll down to the chart originally posted here, the percentages of CRF reports for the 9 sites revealed from the Pfizer document dump are as follows from lowest to highest: 1.4, 2, 2.4, 2.6, 3.1, 3.5, 4.5, 4.7, 6.6. So this means that for the Ventavia sites, for example, if we average out the three percentages, we get a mean of 2.4 which means that up to 2.4% of the participants may have died. For the Sterling Research Group in Mt. Auburn, 6.6% of the participants may have died. At least, a portion of these percentages did die and thus had CRFs filed.
Think about this. Does this make anything better? If the public were indeed left in the dark for 75 years as had been originally requested, then no one would have the opportunity to know that up to 6.6% of the trial participants died (or at least didn’t complete the trial due to an AE) at one of the sites. This is ONLY what has been revealed to date. This is very important to remember.
But let me ask my readers and the public at large: if you knew that, on average (average of the 9/158 sites revealed so far (~6%)), 3.4/100 people died in the trial, would you line up for the shot? Or even worse, 6.6/100? We need them to release this data. All of it.
Greetings all.
This latest data quest literally took me all day and was inspired by a fellow delightfully determined data diver who had been selectively sniffing around ‘CRF data’. I decided to dive back into this continuing saga that is the Pfizer documents, so very slooooooowwwwwwly being released, and take a closer look at this CRF situation. I must say, releasing the data in such an obtuse way, month-by-month, makes it very difficult to assess. A stroke of inane genius. The structure, or lack thereof, the data, and even the file types, also makes analysis extremely difficult.
So, let us return together to the Pfizer documents released by the Public Health and Medical Professionals for Transparency found here.
The reader will note that there are a few listed Case Report Forms (CRFs) for specific sites where the clinical trials were taking place. Sites such as 1081, 1096 and 1128 as listed below.
Now, most of you know that Pfizer is under court order to release their safety and efficacy documents to the public. So far, we, the public, have received a pittance, and what we have received is akin to Enigma. Even Alan Turing would be perplexed. But not really.
What we have been given so far are 10 pages containing a list of 238 documents of varying type and content. Let us now focus only on the files that pertain to sites and the number of ‘Subject No:’s (Subject numbers) per site as organized/revealed here in the ‘interim-mth6-randomization-sensitive’ document which provides a list of randomization numbers for the participants. I will use the words ‘Subjects’ and ‘IDs’ interchangeably.
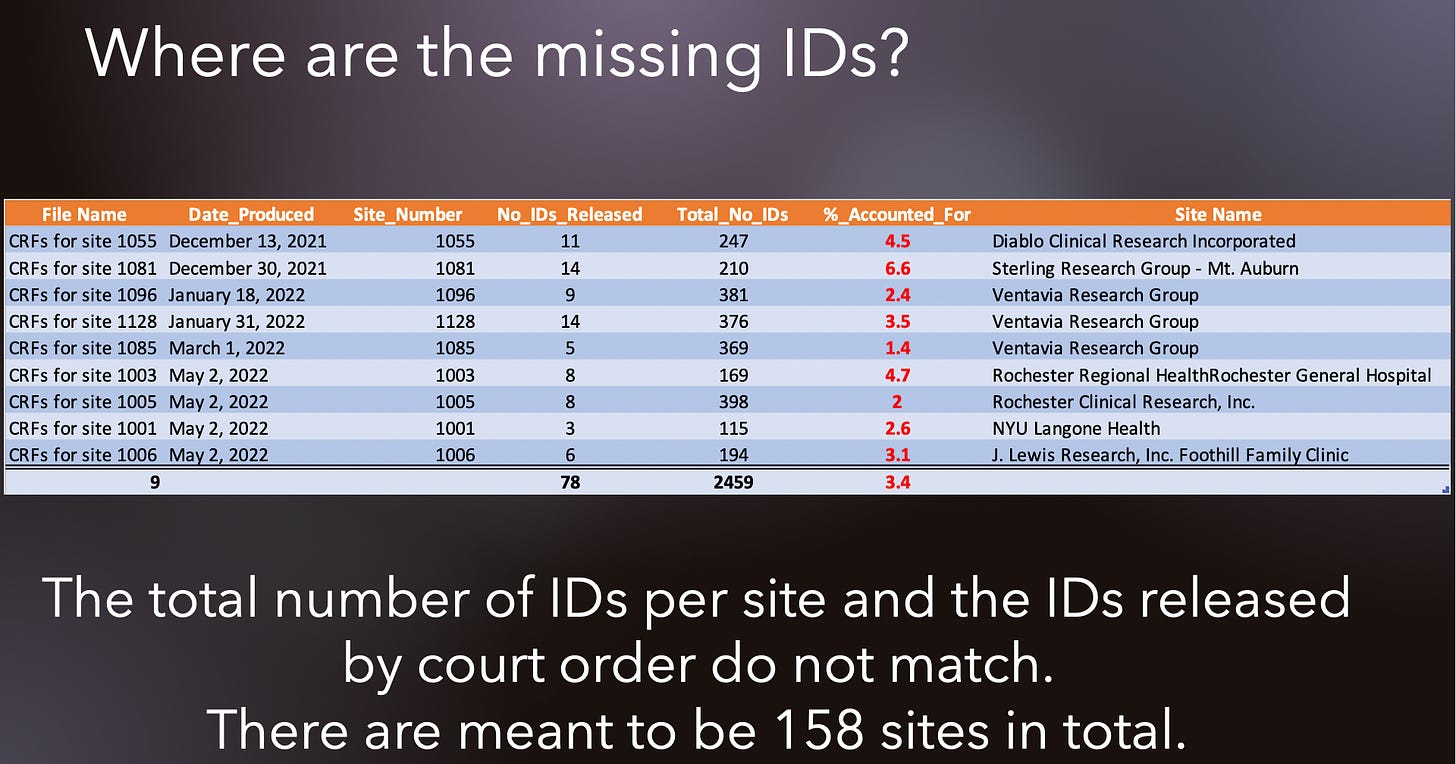
I don’t want to beat around the bush here. There are hundreds of Subject numbers missing if we assume that every participant actually has a CRF written for them. It is my understanding that every trial participant must have a CRF (ICH GCP1 2). I compiled the data and made a comprehensive table to put the totals in one place to show what is missing. There are a total of 9 sites revealed so far (as of May 2, 2022) of 158, by my count from here. Of these sites, in the column labeled Total_No_IDs, are the numbers of Subjects (same as IDs) meant to be accounted for, for a particular site according to this document. As you can see in the column labeled No_IDs_Released, the number of Subjects is much less than what it should be. The percentages of Subjects actually accounted for are all under 6.6%! In the case of the Ventavia Research Group for site 1085, a mere 1.4% of Subjects are accounted for. Since Pfizer is under court order to release complete lists, what on earth is going on here? Are these the complete lists? If these are the complete lists of Subjects, then where did the data relating to the hundreds of other Subjects/participants go?
I literally felt a bit stomach sick going through this today. The number of inconsistencies is so beyond alarming and this is just the tip of the iceberg. And we all know what happened to poor Titanic. I love that movie.
https://www.ich.org/page/efficacy-guidelines
https://ichgcp.net/8-essential-documents-for-the-conduct-of-a-clinical-trial







This is exactly what we should expect from Pfizer, a serial criminal fraud company that has paid billions in fines for its crimes. If they want to take 75 years to release their data then we should wait 75 years to take the toxic jabs.
Unacceptable Jessica is Unstoppable. Keep plugging away.